Essential Electronic Materials: Part 6 - Conductive and Insulating Materials
1 Introduction
In the realm of electronic materials, the distinction between conductive and insulating materials is crucial for the functioning of various electrical and electronic devices. Conductive materials, including metals, alloys, conductive ceramics, and superconductors, are fundamental to efficient energy transmission, high-speed computing, and energy storage. These materials enable the controlled movement of electrons, making them essential for operating electrical circuits and devices. On the other hand, insulating materials play an equally important role in ensuring safety and stability by preventing unwanted flow of electrical currents and protecting electronic components from external influences. This section delves into the essential characteristics, applications, and advancements in conductive and insulating materials, shedding light on their vital roles in modern technology.
2 Conductive Materials
2.1 Metals and Alloys
The high electrical conductivity of metals and alloys stems from their unique structural characteristics. Due to the low ionization energy of metal atoms, their outer electrons (valence electrons) can easily break away from the nucleus to form free electrons, which can move unobstructed in the crystal lattice structure of the metal and thus efficiently conduct electric current. Metal atoms are interconnected by metallic bonds to form dense crystal structures, such as body-centered cubic, face-centered cubic, and hexagonal close-packed structures. This dense arrangement allows the nucleus to form a continuous sea of electrons. The sea is accompanied by a cloud of free electrons, which enhances the electrical and thermal conductivity of the metal. Alloying materials, on the other hand, further optimize their microstructure by introducing different metallic or non-metallic elements into the base metal to form homogeneous or non-homogeneous solid solutions or compounds. Through solid solution strengthening and precipitation strengthening mechanisms, the electrical conductivity of alloys can be modulated, while significantly enhancing their mechanical strength and corrosion resistance, making them adaptable to more complex environments and specialized needs.
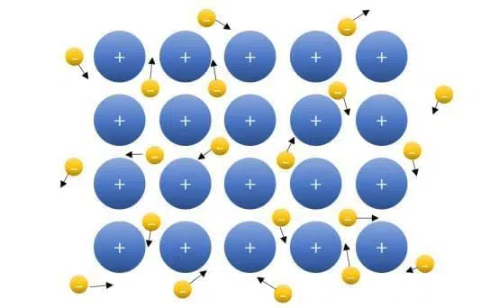
Fig. 1 Free Electrons Can Move Unimpeded Through the Lattice Structure of a Metal
Metals and alloys possess a variety of excellent material properties as conductive materials. Metal conductors such as copper and silver exhibit extremely high conductivity due to their high electron density and low resistivity, while alloy materials usually have slightly lower conductivity than pure metals due to electron scattering caused by doped atoms, but the performance can be enhanced by optimizing the composition. In addition, the thermal conductivity of metals stems from the efficient transfer of heat by free electrons, and highly thermally conductive materials (e.g., copper) are widely used in the field of heat dissipation. Alloys can significantly improve mechanical strength and hardness while maintaining a certain level of electrical conductivity by adjusting their composition and heat treatment. For example, aluminum alloys have better tensile strength and durability compared to pure aluminum and are widely used in aerospace and power transmission applications. Some alloys (such as brass and stainless steel) also have excellent corrosion resistance due to the formation of surface oxide films, while high melting point metals such as tungsten and molybdenum and their alloys can maintain good electrical conductivity and structural stability at high temperatures, which makes them suitable for use in extreme environments for electronic and electrical equipment.
Metals and alloy materials have a wide range of typical applications in the field of electrical conductivity. Copper is widely used in wires, cables, PCB conductors, and heat sinks due to its extremely high electrical and thermal conductivity; silver has the highest electrical conductivity but is more costly and is usually used in high-end electronics, solar cells, and contact point materials; aluminum is mainly used in high-voltage transmission lines and aviation cables due to its lightweight, high electrical conductivity and low cost. In the alloy materials, copper alloys (such as brass, and bronze) with high electrical conductivity and excellent mechanical properties, suitable for electrical contact materials, power switches, and electromagnetic shielding devices; aluminum alloys with lightweight and high strength, widely used in power transmission lines, cable conductors and automotive electronics. Nickel-chromium alloy (Nichrome) is commonly used in heating elements and resistance materials due to its high-temperature stability; tungsten-copper alloy combines the high melting point of tungsten and the high conductivity of copper, which is suitable for high-temperature electrical contacts and rocket engine nozzles; gold alloys are commonly used in semiconductor connecting wires and high-reliability contactors and other high-end fields due to their excellent antioxidant properties and high conductivity.
Fig. 2 Various Metal Wires
2.2 Conductive Ceramics
The electrical conductivity of conductive ceramics stems from their special crystal structure and electron transport mechanism. Some conductive ceramics achieve conductivity through ion migration (e.g. zirconium oxide), while others do so through electron transport (e.g. titanium oxide). By doping specific metals or oxides (e.g., calcium-doped zirconium oxide or tin-doped indium oxide), their conductivity can be significantly altered to increase the concentration of free carriers. In addition, polycrystalline conductive ceramics may have defects at grain boundaries that affect the conductive paths, but their conductivity and mechanical properties can be effectively optimized by high-temperature sintering processes.
Conductive ceramics combine the high-temperature resistance of traditional ceramic materials with the conductive properties of electrically conductive materials, and their performance is characterized by diverse advantages. Conductive ceramics have a wide range of conductivity, from semiconductors to good conductors, and the specific performance by the material composition and the degree of doping is determined. Conductive ceramics maintain stable conductivity at high temperatures and are suitable for extreme conditions. In addition, conductive ceramics exhibit greater corrosion resistance in acidic and alkaline environments compared to metals. Despite their brittleness, their high hardness and compressive strength make them suitable for applications that require them to withstand mechanical stress. Some conductive ceramics (e.g., indium tin oxide, ITO) also combine transparency and conductivity, making them ideal for optoelectronic devices.
Conductive ceramics have a wide range of applications in electronics, energy, and sensing. In electronics and optoelectronics, indium tin oxide (ITO) is widely used as a transparent electrode for touch screens, LCDs, and OLED screens due to its transparent conductivity, while titanium oxide (TiO2) is used in solar cells, photocatalytic devices, and sensors. In the energy sector, calcium-doped zirconia (CaZrO3) is used as an electrolyte material in solid oxide fuel cells (SOFC), while zinc oxide (ZnO) is used in varistors and transparent conductive films. For high temperatures and extreme environments, silicon carbide (SiC) and silicon nitride (Si3N4) are suitable for manufacturing high-temperature electronics, high-frequency devices, and aerospace components. In addition, conductive ceramics are widely used in gas sensors (e.g. oxygen sensors) and thermistors, and antistatic protection is realized in electronic devices by coatings made of conductive ceramic powders. These diverse applications demonstrate the importance of conductive ceramics in modern technology.
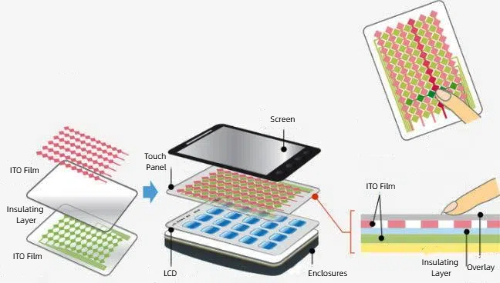
Fig. 3 ITO Film for Touch Panels
2.3 Conductive Glass
The structure of conductive glass usually consists of a highly transparent glass substrate compounded with a conductive film on the surface, whose conductivity is mainly derived from the transparent conductive oxide (TCO) film covering the surface. The glass substrate is generally made of soda-lime or quartz glass, which provides excellent mechanical strength and optical properties; the conductive film is made of common materials including indium tin oxide (ITO), fluorine-doped tin oxide (FTO), and aluminum-doped zinc oxide (AZO), which are deposited by processes such as vacuum sputtering or chemical vapor deposition (CVD), and the thickness of the film is usually tens of nanometers to hundreds of nanometers. In addition, by doping the oxides with specific elements (e.g., tin, aluminum, or fluorine), the carrier concentration can be significantly increased, enhancing the conductivity of the films.
Conductive glass combines optical transparency with electrical conductivity and exhibits a variety of excellent properties. Its conductive film of visible light transmittance of up to 80% or more, while maintaining a low reflectivity, to achieve high light transmittance; film conductivity is good, the resistivity is usually between 10^-3 to 10^-4 Ω-cm, can meet the needs of most electronic devices. The glass substrate has high mechanical strength and heat resistance, and the film is firmly bonded to the substrate and can be used at certain high temperatures. Conductive films are also resistant to oxidation and corrosion, making them suitable for long-term exposure to the environment. In recent years, flexible conductive glass has become a research hotspot, further expanding its application scenarios through designs based on plastic or ultra-thin glass.
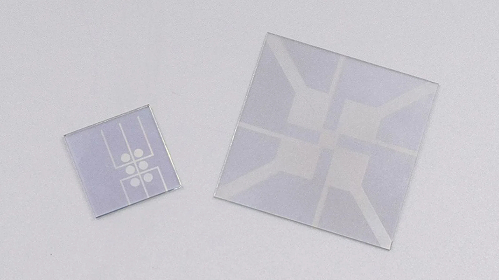
Fig. 4 ITO Conductive Glass
Conductive glass is widely used in several fields due to its unique properties. In photovoltaic power generation, it is used as a transparent electrode in solar cells (e.g., silicon solar cells and chalcogenide solar cells) to ensure efficient light absorption and charge collection efficiency; in display technology, it is used as a transparent conductive layer in liquid crystal displays (LCDs), organic light-emitting diode (OLED) screens, and touchscreens. In smart windows and light-control devices, conductive glass is used in electrochromic windows, light-control mirrors, and smart shading devices for energy management and privacy protection. It is also widely used in the manufacture of electronic devices such as gas sensors, flexible electronics, heating glass, and anti-condensation glass. In the field of optical and communication devices, conductive glass is used as a transparent conductive dielectric layer in optical thin film devices and laser communication systems.
2.4 Superconducting Material
Superconducting materials are a class of materials whose resistance drops to zero at a specific temperature and exhibits complete anti-magnetism. According to their critical temperature and structural properties, they can be divided into the following categories: low-temperature superconducting materials (LTS), such as niobium (Nb), niobium-titanium alloys (Nb-Ti), and niobium-tris-tin (Nb3Sn), which have low critical temperatures (usually below 30 K) and require liquid helium or liquid nitrogen for cooling and are widely used in strong-field devices such as magnetic resonance imaging (MRI) and particle accelerators. Imaging (MRI) and particle gas pedals and other strong magnetic field equipment; high-temperature superconducting materials (HTS), such as yttrium barium copper-oxygen (YBCO) and bismuth strontium calcium copper-oxygen (BSCCO), with critical temperatures exceeding 77 K, which can be cooled by liquid nitrogen, greatly reducing the operating cost, and are suitable for electric power transmission and high-temperature magnetic levitation; iron-based superconducting materials, including iron selenium (FeSe) and iron arsenide (LaFeAsOx) ₋xFx), with structural stability and strong antimagnetism, are promising for high magnetic field devices and future electronic components; organic superconducting materials, such as those based on fullerenes (C60) or aromatic compounds, despite the lower critical temperature, are flexible and lightweight, suitable for flexible electronic devices; topological superconducting materials, combining superconductivity with topological properties, such as certain topological insulators and epitaxial thin-film materials, with possible applications in quantum computing and topological electronics.
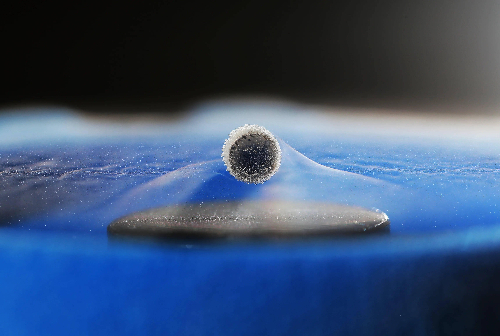
Fig. 5 Superconducting Material
Superconducting materials exhibit the following unique properties in electrical applications: firstly, zero resistance is its core property. In the superconducting state, the resistance is completely zero, and the current can flow through the superconductor without loss, thus greatly reducing energy consumption, which is particularly suitable for long-distance power transmission and high-efficiency energy storage. Secondly, superconducting materials exhibit complete antimagnetism (Meissner effect), i.e., in the superconducting state the internal magnetic field is completely repelled, allowing the magnetic lines of force to bypass the surface of the superconductor. This property enables superconductors to achieve stable levitation and is therefore widely used in the field of magnetic levitation trains and frictionless bearings. The critical temperature (Tc) of a superconducting material determines the temperature at which it needs to be cooled to enter the superconducting state, which varies significantly from material to material, e.g., low-temperature superconducting materials need to be cooled by liquid helium, while high-temperature superconducting materials can be cooled by liquid nitrogen, which greatly reduces operating costs. Critical magnetic field (Hc) and critical current density (Jc) are important parameters limiting the performance of superconductivity. When the strength of the external magnetic field or the current density through the superconductor exceeds the critical value, the superconducting state will be destroyed. Materials with high critical parameters are more suitable for strong magnetic field environments and high current devices such as magnetic resonance imaging (MRI) and particle gas pedals. In addition, superconductors exhibit the Josephson effect, a tunneling current formed between superconductors through insulators. This effect has important applications in ultra-high sensitivity magnetic sensors, superconducting quantum interference devices (SQUIDs), and quantum computing. These properties give superconducting materials great potential for efficient energy transfer, strong magnetic field applications, and cutting-edge technologies.
Superconducting materials are used in a wide range of applications due to their unique electrical properties. In the field of power and energy, superconducting cables utilize zero resistance to achieve long-distance transmission and significantly reduce energy loss; superconducting generators improve energy efficiency and reduce size and weight; and superconducting energy storage systems (SMES) can store and release large amounts of energy in a short period for grid regulation and stabilization. In medical and scientific research, magnetic resonance imaging (MRI) equipment utilizes low-temperature superconductors to generate strong magnetic fields, superconducting magnets are used in particle gas pedals (e.g., the LHC) to generate strong magnetic fields, and superconducting quantum interferometers (SQUIDs) are used in magnetoencephalography and geomagnetic probing as highly sensitive magnetic field sensors. In transportation and engineering, superconducting maglev trains utilize anti-magnetism for frictionless high-speed transportation and high-temperature maglev bearings are used for contactless rotating parts in aerospace and industrial machinery. In information technology, superconducting materials are at the core of quantum computing, and superconducting quantum bits based on the Josephson effect have boosted quantum computing research; in addition, superconducting electronic devices such as superconducting filters and high-frequency amplifiers are widely used in communications and signal processing. In the military and aerospace fields, superconducting electromagnetic guns utilize strong superconducting magnets to achieve efficient acceleration, while superconducting radar enhances signal sensitivity and detection accuracy. These applications demonstrate the great potential of superconducting materials in the fields of energy, medicine, transportation, information technology, and defense.
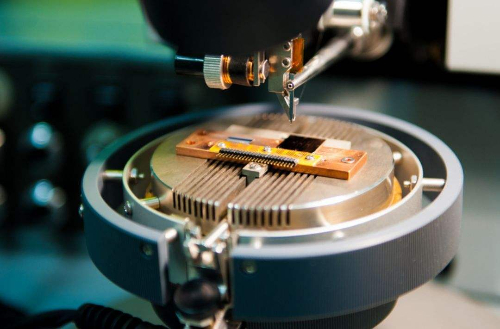
Fig.6 Application of Superconducting Material
3 Insulating Materials
3.1 Inorganic Insulating Materials
Inorganic insulators are a class of materials with high electrical resistivity and good heat resistance and are widely used in the field of electrical insulation. Typical inorganic insulators include glass, ceramics, and mica. Glasses are mainly composed of silicates (SiO2), whose interiors form a stable amorphous structure by covalently bonded silica-oxygen tetrahedra that prevent the movement of free electrons. Ceramics are usually composed of materials such as alumina (Al2O3) and zirconia (ZrO2), which form a dense crystalline structure with very low electron mobility and ionic conductivity. Mica, on the other hand, consists of a silicate layered structure with weak bonding between the layers, which makes it easy to process into thin sheets and exhibits excellent electrical insulation and thermal stability.
Inorganic insulating materials have a series of excellent properties that make them widely used in the field of electrical insulation. First, they have high resistivity, usually above 10^12 Ω-cm, which can effectively stop current leakage and ensure the safe and stable operation of electrical equipment. Secondly, good heat resistance is an important feature of inorganic insulators. Glass and ceramics can withstand high temperatures ranging from hundreds to thousands of degrees Celsius, while mica maintains stable electrical properties at high temperatures. High mechanical strength is also a significant advantage of inorganic materials, ceramics and glass have high hardness and wear resistance, suitable for applications subjected to large mechanical stress; while mica flakes have a certain degree of flexibility, easy to process into a variety of shapes. Inorganic insulating materials are also chemically resistant, able to resist the erosion of acids, alkalis, and moisture, showing good durability in harsh environments. Finally, excellent dielectric properties and high dielectric strength of inorganic insulators can withstand high voltage without breakdown, to ensure safety in high-voltage environments.
Inorganic insulating materials have a wide range of applications in several fields. Glass is mainly used for high-voltage insulators in electrical equipment and the housings of vacuum circuit breakers, in addition to being used as an encapsulating material for electronic equipment to protect components. Ceramics are widely used as insulators in transformers and switchgear, providing good dielectric properties and heat dissipation. They are also used as substrates for high-frequency equipment and to make spark plug insulators and insulating components for high-voltage lines. Mica, on the other hand, is commonly used as an insulating sheet for electric motors and generators, capable of withstanding high temperatures and pressures. In addition, it is used to insulate electric heating elements in heating equipment and as an insulating material for capacitors in high-frequency circuits, providing a low-loss dielectric.

Fig. 7 Inorganic Mineral Insulation
3.2 Polymer Insulation
Polyvinyl chloride (PVC) is a polymer compound formed by the polymerization of vinyl chloride monomer, with linear or branched carbon-chlorine chain structure and strong chemical stability. It has good electrical insulation and high electrical resistivity, which prevents leakage of electric current. PVC is also highly resistant to chemicals such as acids, alkalis, and salts, as well as to abrasion and processing, making it suitable for mass production. However, its heat resistance is average, and it is usually suitable for temperatures ranging from -10°C to 60°C. PVC is widely used in the outer sheath of cables and wires, and the insulation and protection of electrical equipment, and is particularly suitable for low-voltage applications.
Polyimide (PI) is a polymer material with a rigid ring structure, the main chain consists of imide groups (-C=O-N-), exhibiting high mechanical strength and heat resistance. Polyimides are extremely heat resistant and can be used for long periods at high temperatures, ranging up to 250°C or even higher. Its excellent electrical insulation makes it particularly suitable for high-voltage and high-frequency electrical equipment. Polyimide also has good mechanical strength, abrasion resistance, and excellent chemical stability, and can withstand most chemical solvents. Common applications include high-temperature cables, electrical equipment in aerospace, printed circuit boards (PCBs), and insulation for electronic components.
Polytetrafluoroethylene (PTFE) is a linear polymer material formed by the polymerization of tetrafluoroethylene monomers. The strong electronegativity of the fluorine atom allows it to exhibit extremely low coefficients of friction and excellent chemical stability. PTFE has an extremely low dielectric constant and outstanding electrical insulation, enabling it to be used in high-frequency and high-voltage environments. It has very high chemical resistance to virtually all chemicals, including strong acids, bases, and solvents. PTFE also has good heat resistance and can be used in temperatures ranging from -200°C to 260°C, while exhibiting excellent abrasion resistance and low friction characteristics. Common applications include high-voltage cables, insulation protection for electronic components, lining materials for chemical piping, and insulation needs for work in extreme environments (such as high temperature, strong acid or alkali environments).
Fig. 8 Plastics Used as Wire Packaging
4 Conclusion
The materials discussed—conductive and insulating—serve complementary yet distinct roles in the design and functionality of electronic devices. Conductive materials, from metals like copper and silver to innovative superconductors, offer remarkable electrical conductivity, mechanical strength, and thermal management properties, making them indispensable in power transmission, communication technologies, and high-performance devices. Conversely, insulating materials, such as inorganic materials like ceramics and polymers like PTFE, provide essential electrical isolation, thermal resistance, and mechanical durability. These materials ensure the protection, efficiency, and longevity of electronic systems. As advancements continue in material science, the evolution of both conductive and insulating materials will further optimize the performance of next-generation electronic systems, driving innovation across industries such as energy, communication, healthcare, and aerospace.
Stanford Advanced Materials (SAM) is a key provider of high-quality conductive and insulating materials, supporting these critical applications with reliable material solutions.
Further Reading:
Essential Electronic Materials: Part 1 - Silicon
Essential Electronic Materials: Part 2 - Silicon Carbide
Essential Electronic Materials: Part 3 - Germanium
Essential Electronic Materials: Part 4 - Gallium Compounds
Essential Electronic Materials: Part 5 - Carbon-Based Materials