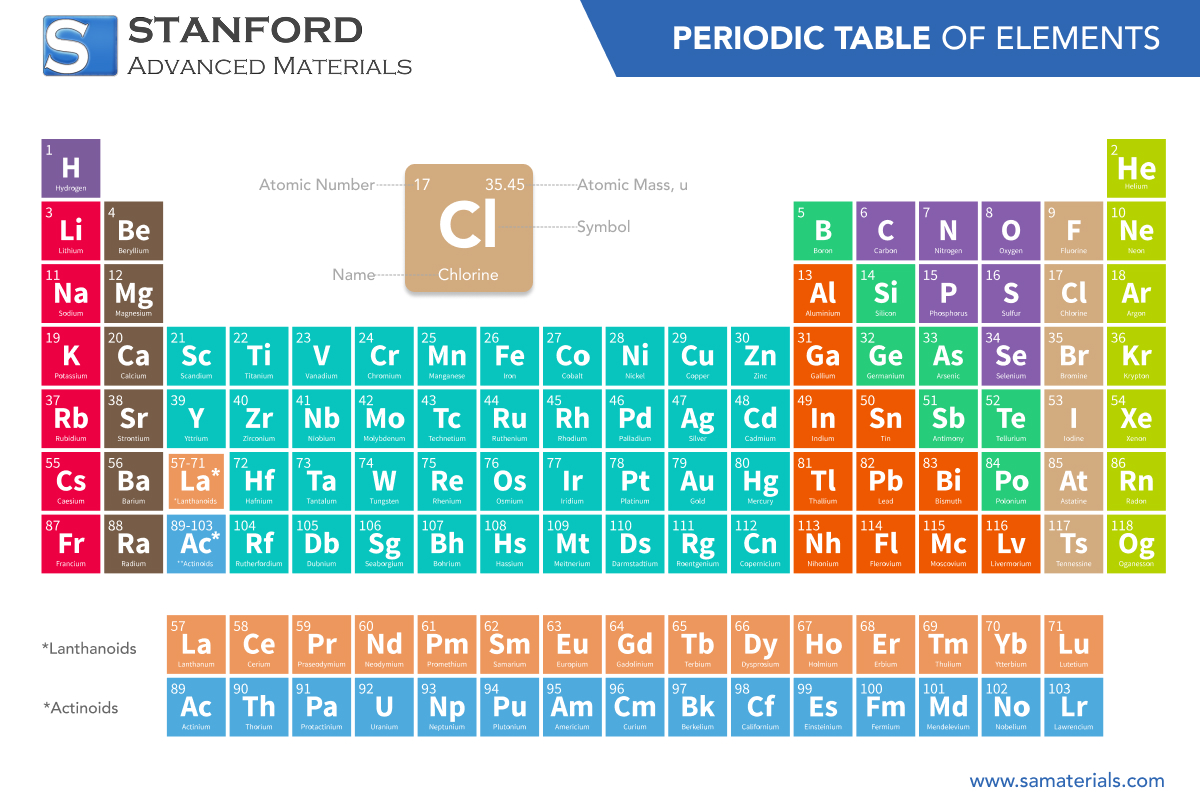
Periodic Table of the Elements
Periodic Table: Introduction
The periodic table categorizes elements based on their atomic number and electron arrangement. Rows represent electron energy levels, while columns group elements with similar properties. Key groups include alkali metals, alkaline earth metals, transition metals, halogens, and noble gases. It's crucial in chemistry for predicting element properties and reactions.
Periodic Table: The Elements
Element | Name | Atomic Number |
Ac | Actinium | 89 |
Silver | 47 | |
Aluminum | 13 | |
Am | Americium | 95 |
Ar | Argon | 18 |
Arsenic | 33 | |
At | Astatine | 85 |
Gold | 79 | |
Boron | 5 | |
Barium | 56 | |
Beryllium | 4 | |
Bismuth | 83 | |
Bromine | 35 | |
Carbon | 6 | |
Calcium | 20 | |
Cadmium | 48 | |
Ce | Cerium | 58 |
Cl | Chlorine | 17 |
Cobalt | 27 | |
Chromium | 24 | |
Cesium | 55 | |
Copper | 29 | |
Dy | Dysprosium | 66 |
Er | Erbium | 68 |
Eu | Europium | 63 |
Fluorine | 9 | |
Iron | 26 | |
Fr | Francium | 87 |
Gallium | 31 | |
Gd | Gadolinium | 64 |
Germanium | 32 | |
Hydrogen | 1 | |
He | Helium | 2 |
Hafnium | 72 | |
Hg | Mercury | 80 |
Ho | Holmium | 67 |
Iodine | 53 | |
Indium | 49 | |
Iridium | 77 | |
Potassium | 19 | |
Krypton | 36 | |
La | Lanthanum | 57 |
Lithium | 3 | |
Lu | Lutetium | 71 |
Magnesium | 12 | |
Manganese | 25 | |
Molybdenum | 42 | |
Nitrogen | 7 | |
Sodium | 11 | |
Niobium | 41 | |
Nd | Neodymium | 60 |
Ne | Neon | 10 |
Nickel | 28 | |
Np | Neptunium | 93 |
O | Oxygen | 8 |
Os | Osmium | 76 |
Phosphorus | 15 | |
Pa | Protactinium | 91 |
Lead | 82 | |
Palladium | 46 | |
Pm | Promethium | 61 |
Po | Polonium | 84 |
Pr | Praseodymium | 59 |
Platinum | 78 | |
Pu | Plutonium | 94 |
Ra | Radium | 88 |
Rubidium | 37 | |
Rhenium | 75 | |
Rhodium | 45 | |
Rn | Radon | 86 |
Ruthenium | 44 | |
Sulfur | 16 | |
Antimony | 51 | |
Scandium | 21 | |
Selenium | 34 | |
Silicon | 14 | |
Sm | Samarium | 62 |
Tin | 50 | |
Strontium | 38 | |
Tantalum | 73 | |
Tb | Terbium | 65 |
Tc | Technetium | 43 |
Tellurium | 52 | |
Th | Thorium | 90 |
Titanium | 22 | |
Tl | Thallium | 81 |
Tm | Thulium | 69 |
U | Uranium | 92 |
Vanadium | 23 | |
Tungsten | 74 | |
Xe | Xenon | 54 |
Yttrium | 39 | |
Yb | Ytterbium | 70 |
Zinc | 30 | |
Zirconium | 40 |
Periodic Table: FAQs
1. Who created the periodic table?
The modern periodic table is credited to Dmitri Mendeleev, a Russian chemist. He arranged elements by increasing atomic mass and observed patterns in their properties, leaving gaps for undiscovered elements.
2. How are elements arranged on the periodic table?
Elements are arranged by increasing atomic number (number of protons) from left to right and top to bottom. Elements with similar properties are placed in the same columns (groups), while rows (periods) signify electron shell levels.
3. What do the rows and columns represent?
Rows, or periods, represent the number of electron shells an element's atoms possess. Columns, or groups/families, indicate elements with similar valence electron configurations and chemical properties.
4. What are some key properties of groups on the periodic table?
l Group 1: Alkali Metals - Highly reactive metals.
l Group 2: Alkaline Earth Metals - Less reactive metals.
l Group 17: Halogens - Highly reactive nonmetals.
l Group 18: Noble Gases - Generally inert gases.
5. What are transitional metals?
Transitional metals are elements found in groups 3-12. They often exhibit variable oxidation states and are good conductors of electricity.
6. Are there elements that are exceptions or don't fit neatly into the table?
Yes, elements called metalloids (e.g., silicon, boron) have properties between metals and nonmetals and are placed along the staircase line on the periodic table.
8. How many elements are currently known?
118 elements are known, with elements beyond uranium (element 92) being synthetic and created in laboratories.
9. Why is the periodic table important?
It's a fundamental tool in chemistry, helping predict an element's properties and behavior based on its position. It allows scientists to understand and organize elements according to their characteristics.