Essential Electronic Materials: Part 2 - Silicon Carbide
1 Introduction
Silicon carbide (SiC) is a broadband semiconductor material with excellent properties of high hardness, high thermal conductivity, high temperature, and corrosion resistance. In the electronics field, SiC is widely used in power electronics, especially in electric vehicles, 5G communications, photovoltaic power generation, and aerospace, due to its ability to work stably at high temperatures, high pressures, and high frequencies. Compared with traditional silicon materials, silicon carbide enables more efficient energy conversion, lower power consumption, and longer device life, making it an ideal choice for high-performance electronic devices.
2 Basic Properties of Silicon Carbide
Silicon carbide, an inorganic substance with the chemical formula SiC, is smelted at high temperatures in resistance furnaces using raw materials such as quartz sand, petroleum coke (or coal coke), and wood chips (table salt is added to produce green silicon carbide). Silicon carbide is a semiconductor in nature in the form of the extremely rare mineral moissanite. It has been mass-produced as powder and crystals since 1893 and is used as an abrasive. Among the non-oxide high-tech refractory raw materials such as C, N, B, etc., silicon carbide is the most widely and economically used and can be referred to as golden steel sand or refractory sand.
Fig. 1 Silicon Carbide Wafer
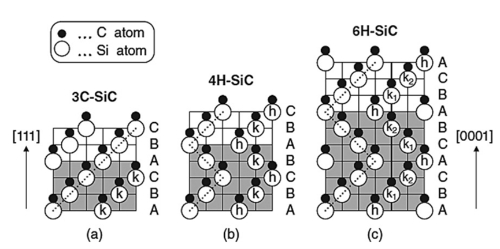
Black silicon carbide and green silicon carbide, two commonly used varieties, are α-SiC. Black silicon carbide contains SiC of about 95%, and its toughness is higher than the green silicon carbide, mostly used in the processing of low tensile strength materials, such as glass, ceramics, stone, refractory materials, cast iron, and non-ferrous metals. Green silicon carbide containing SiC of about 97% or more, self-sharpening good, mostly used in the processing of cemented carbide, titanium alloy, and optical glass, also used for honing cylinder liner and precision grinding of high-speed steel cutting tools. In addition, there is cubic silicon carbide, it is a special process of yellow-green crystals, used to make the abrasive suitable for ultra-fine machining of bearings, which can make the surface roughness from Ra32 ~ 0.16 microns to Ra0.04 ~ 0.02 microns of one-time processing.
SiC is a typical binary compound semiconductor material, and the basic unit of its crystal structure is a tetrahedron with fourfold symmetry, i.e., SiC4 or CSi4, where the distance between two adjacent Si atoms or two C atoms is 3.08 Å, and the distance between adjacent C and Si atoms is only about 1.89 Å. In SiC crystals, Si and C atoms form very strong tetrahedral covalent bonds (bonding energy of 4.6 eV) by sharing electron pairs on sp3 hybridized orbitals. Sharing electron pairs on the sp3 hybridized orbitals to form very strong tetrahedral covalent bonds (bond energy of 4.6 eV).
Pure silicon carbide is a colorless and transparent crystal. Industrial silicon carbide is light yellow, green, blue, or even black depending on the type and content of impurities, and its transparency varies with its purity. Silicon carbide crystal structure is divided into hexagonal or rhombic α-SiC and cubic β-SiC (called cubic silicon carbide). α-SiC due to its crystal structure of carbon and silicon atoms in the stacking of different sequences and constituting several different variants, has been found in more than 70 kinds of. β-SiC in 2100 ℃ or more when the transformation of α-SiC. α-SiC is the most common type of crystal, β-SiC is the cubic crystal system, also known as cubic silicon carbide. It is also known as cubic silicon carbide. Until now, β-SiC has had relatively little commercial use, although it can be used as a carrier for multiphase catalysts due to its higher surface area than α-SiC. Silicon carbide is industrially manufactured by refining it in a resistance furnace using high-quality quartz sand and petroleum coke. The refined silicon carbide blocks are crushed, acid and alkali-washed, magnetically selected, and screened or water-selected to make products of various particle sizes.
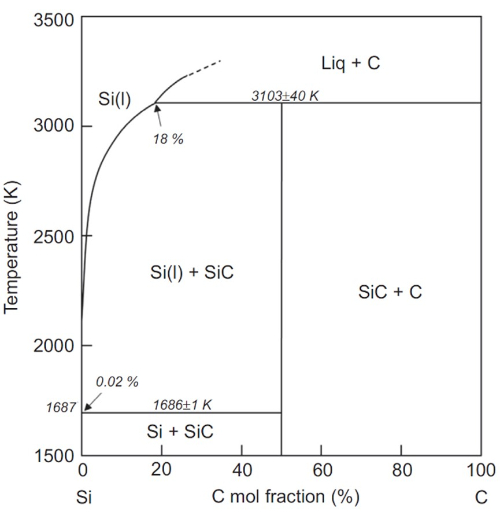
Fig. 2 Binary phase diagram of SiC
Due to its stable chemical properties, high thermal conductivity, low coefficient of thermal expansion, and excellent wear resistance, silicon carbide has many applications beyond abrasives. For example, applying silicon carbide powder to the impeller of hydraulic turbines or the inner wall of cylinders through a special process can enhance abrasion resistance and extend service life by 1 to 2 times. Additionally, silicon carbide is used in high-grade refractory materials, offering benefits such as thermal shock resistance, compact size, lightweight, and high strength, making it highly energy-efficient. Low-grade silicon carbide (around 85% SiC) is an excellent deoxidizer that can accelerate steelmaking, facilitate chemical composition control, and improve steel quality. Additionally, silicon carbide is widely used in the production of silicon carbon rods for electric heating elements.
Silicon carbide hardness is very large, Mohs hardness of 9.5, second only to the world's hardest diamond (10), has excellent thermal conductivity, and is a semiconductor, with high-temperature oxidation resistance.
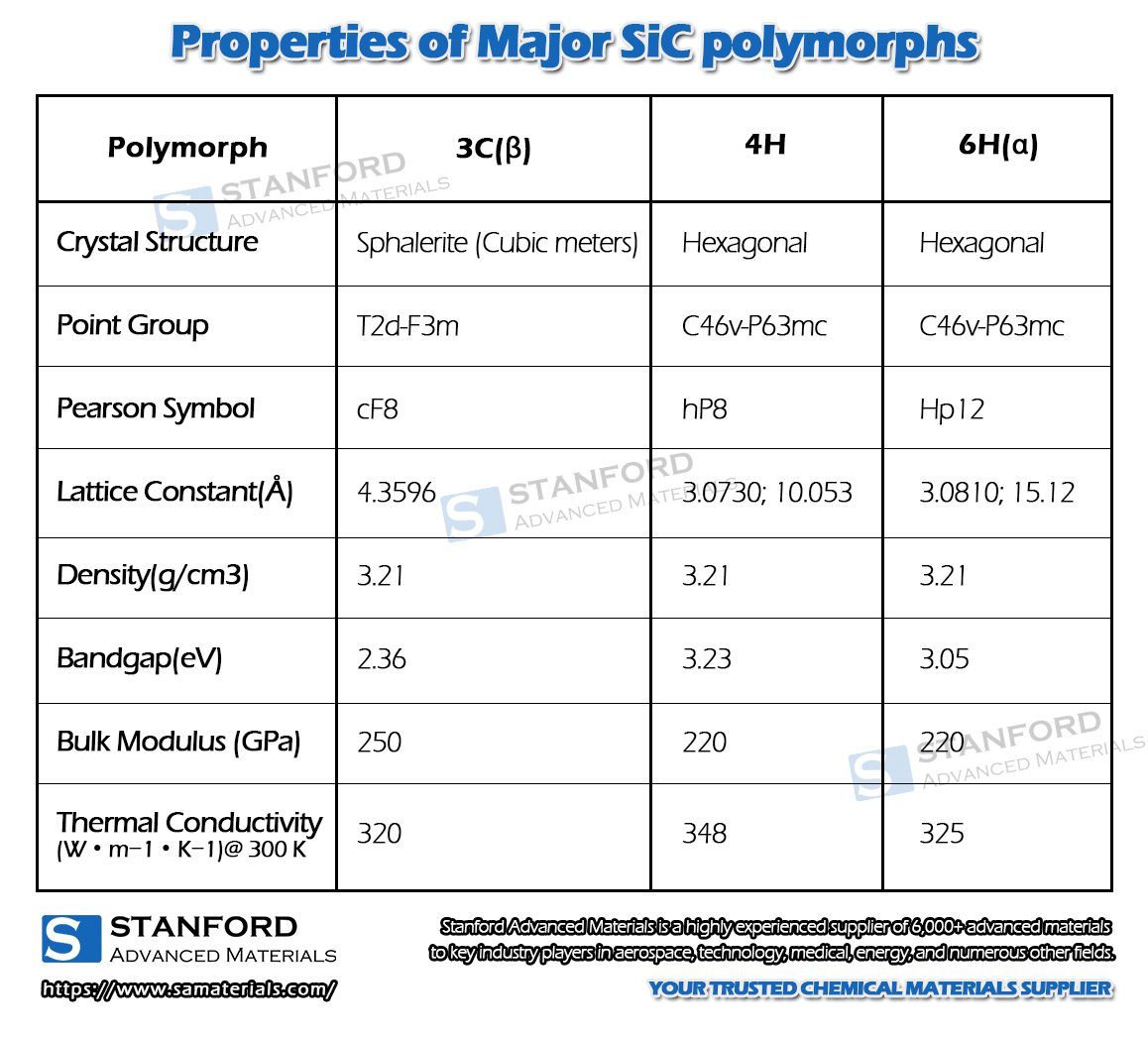
There are at least 70 crystalline forms of silicon carbide. α-SiC is the most common homogeneous heterocrystal, formed at high temperatures above 2000 °C and has a hexagonal crystal system (resembling fibrous zincite). β-SiC, with a cubic crystal system, is similar to diamond and is produced at temperatures below 2000 °C. β-SiC is a heterogeneous catalyst carrier with a higher specific surface area than α-SiC. For heterogeneous catalyst carrier applications, β-silicon carbide is of interest due to its higher specific surface area than α-silicon carbide. There is another type of silicon carbide, μ-silicon carbide, which is the most stable and produces a more pleasant sound on impact. So far, however, these two types of silicon carbide have not been used commercially.
Fig. 3 Crystal structures of major SiC polymorphs
Due to its specific gravity of 3.1 g/cm3 and its relatively high sublimation temperature (approx. 2700 °C), silicon carbide is well suited as a raw material for bearings or high-temperature furnaces. It does not melt at any attainable pressure and has a rather low chemical activity. Because of its high thermal conductivity, its high collapsing electric field strength, and the fact that it possesses the highest current density, there have been attempts to use silicon carbide as a replacement for silicon, especially in semiconductor high-power component applications. In addition, silicon carbide is strongly coupled to microwave radiation and, because of its high sublimation point, allows it to be applied to heat metals.
Pure silicon carbide is colorless, but in industrial production, its color is usually brown to black due to the presence of impurities such as iron. The iridescent luster of the crystal surface is due to the formation of a protective layer of silica.
SiC is a semiconductor that, through doping changes the energy level structure of SiC materials, and further modulates its properties, mainly using ion implantation means for A, B, N, and other atoms of the doping. Among them: Al and other host atoms are more likely to take the place of Si in the SiC lattice to form a deeply dominant energy level, thus obtaining a P-type semiconductor; while N and P and other host atoms are more likely to occupy the lattice position of C to form a shallow dominant level, thus obtaining an N-type semiconductor. It is worth noting that SiC has a wide doping range (1X1014-1X1019 cm-3) not found in other wide bandgap semiconductors, and it is easy to realize N-type and P-type doping in this range, e.g., the electrical resistivity of 4H-SiC single crystals is as low as 5 Ω-cm after doping with AI.
3 Silicon Carbide Fabrication Processes
Silicon carbide is produced by two main methods: the fusion method and the chemical vapor deposition method.
3.1 Fusion Method
The fusion method is to melt silicon and graphite (or graphitized silicon) by mixing them at a high temperature and then cooling them to form silicon carbide. The specific process is as follows:
1. Raw material preparation: select high-purity carbon raw materials and silicon raw materials, and pulverize, and sieve, so that the particle size meets the process requirements.
2. Mixing: Mix the crushed carbon and silicon raw materials according to a certain ratio, so that the impurities are dispersed.
3. Charging: the mixed raw materials into the high-temperature furnace, the furnace should be fixed in a certain furnace temperature, and atmosphere and maintain a certain negative pressure.
4. Carbonization reaction: at a high temperature, carbon and silicon raw materials react to produce silicon carbide. The temperature of the reaction is usually between 2000-2500 degrees Celsius.
5. Cooling and separation: After the carbonization reaction, the furnace is closed for cooling. The silicon carbide material is then removed from the furnace and the silicon carbide of different particle sizes is separated by physical methods (e.g. crushing, sieving).
3.2 Chemical Vapor Deposition
Chemical vapor deposition (CVD) is a method of forming silicon carbide on the surface of a substrate by depositing a carbon and silicon source in a gas through a gas-phase chemical reaction. The specific process is as follows:
1. Substrate preparation: choose suitable substrates, such as quartz, graphite, etc., and clean and treat them under the requirements of the process to make the surface smooth.
2. Reactor loading: put the treated substrate into the CVD reactor, and heat the reactor to a suitable temperature.
3. Reaction gas supply: Supply the gas containing carbon and silicon sources into the reactor at a certain flow rate, and control the reaction temperature, pressure, and gas ratio at the same time.
4. Gas phase reaction: The gases from carbon and silicon sources react chemically on the surface of the substrate to produce silicon carbide. The nature of the silicon carbide can also be changed by introducing doping sources during the reaction process.
5. Cooling and curing: After the reaction is finished, the gas supply is stopped, the reactor is shut down, and cooling takes place. During the cooling process, the silicon carbide cures on the surface of the substrate to form a thin film or block of silicon carbide.
Depending on the application requirements, a suitable process can be selected to produce silicon carbide materials with specific properties.
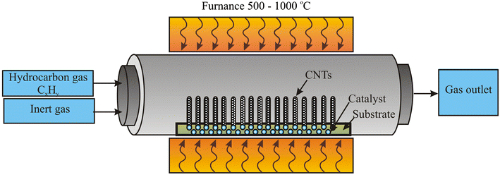
Fig. 4 Facility of Chemical Vapor Deposition (CVD)
4 Applications of Silicon Carbide
4.1 Power Electronics
In power semiconductor devices (e.g., MOSFETs, IGBTs), silicon carbide offers a more efficient solution for power conversion. While conventional silicon materials have limited performance at high currents and high voltages, SiC materials have wide-bandwidth characteristics that enable them to maintain low switching losses and reduce energy loss at high voltages. This advantage is particularly prominent in electric vehicles and renewable energy generation systems, providing longer range and shorter charging times for electric vehicles, as well as improved energy efficiency in photovoltaic and wind power systems. In addition, silicon carbide enables efficient energy conversion in high-voltage devices in power grid systems, supporting intelligent and efficient power transmission.
4.2 High Temperature and High Frequency Devices
Silicon carbide excels in the high-temperature and high-frequency fields and is particularly suitable for high-frequency switches that require high-speed operation in 5G base stations and military electronics. Its ability to maintain stable electrical performance in high-temperature environments makes up for the lack of performance degradation of traditional silicon devices under high-temperature conditions. Due to its wide bandwidth and high thermal conductivity, SiC can maintain good electrical performance at higher operating temperatures than silicon, making it an ideal material for high-frequency communication and radar systems, and able to meet the demand for higher data transmission rates in 5G base stations.
4.3 LED and Optoelectronic Applications
Silicon Carbide was an early material used for blue LEDs. Although it is now often replaced by gallium nitride, it remains valuable in optoelectronic devices for specific wavelength bands, particularly in UV and NIR photodetection. SiC's high-temperature and radiation-resistant properties allow it to be used in a wide range of applications in extreme optical environments. As a stable substrate, it can be integrated into high-temperature and radiation-resistant photodetectors suitable for lighting systems and optical sensor devices that require high light output stability.
Fig. 5 Silicon Carbide Wafer for Optoelectronic Applications
4.4 Sensors
SiC shows unique advantages in extreme environment sensors. It can accurately detect gas, temperature, pressure, and other parameters in chemical sensors and high-temperature gas sensors, which is suitable for petrochemical and other industries that require high chemical stability. SiC sensors have excellent corrosion resistance and high-temperature stability and can operate effectively in environments where traditional sensors fail, which is especially suitable for high-temperature and highly corrosive industrial environments.
4.5 Aerospace and Defense Applications
In aerospace and defense equipment such as satellites and missiles, which require high reliability and durability, Silicon Carbide is favored for its high melting point, radiation resistance, and strength characteristics. SiC devices not only withstand extreme temperatures but also provide reliable performance in vacuum and strong radiation environments, allowing them to play a central role in critical areas such as satellite communications and missile control, enhancing the reliability and service life of equipment in extreme environments.

Fig. 6 Silicon Carbide Mirrors for Aerospace Applications
5 Advantages and Limitations of Silicon Carbide
5.1 Advantages of Silicon Carbide
1. High-temperature and high-pressure performance: SiC's thermal stability and electrical properties in high-temperature environments are superior to those of traditional silicon materials. The high melting point and antioxidant properties of SiC make it stable in extreme environments, which is especially important in aerospace, military electronic equipment, and other applications that require high-temperature resistance. Its wide bandwidth structure has a lower leakage current at high voltages, which greatly reduces the impact of thermal effects, an advantage that is difficult to achieve with traditional silicon materials.
2. High-frequency and high-power applications: The wide-bandwidth characteristics and high carrier mobility of SiC materials give them a significant advantage in high-frequency and high-power applications. Compared to silicon, SiC devices can more effectively reduce energy loss in high-frequency devices and perform superiorly in high-power applications. This makes it an ideal material for electronic devices that require high-speed signal switching, such as 5G communication base stations and high-frequency radar systems.
3. Efficient energy conversion: SiC power devices can significantly improve energy efficiency in electric vehicles and renewable energy systems. Its low conduction loss and high voltage blocking ability, so that the SiC inverter in electric vehicles more efficient, effectively extending the range time. In addition, in solar and wind energy system inverters, SiC devices also significantly improve the energy conversion efficiency, reduce the system operating temperature, and improve the reliability of the equipment.

Fig. 7 High-Temperature Silicon Carbide Reduction Tank
5.2 Limitations of Silicon Carbide
1. High cost: Compared to silicon materials, SiC is more expensive to prepare and process, and requires specific high-precision process support, which has a greater impact on production costs. The growth cost of high-quality SiC crystals is much higher than that of silicon materials, so the market price of SiC devices is still high, affecting its promotion in cost-sensitive markets such as consumer electronics.
2. Complex process: The SiC single crystal preparation process is complex, especially when preparing high-purity, high-quality SiC single crystal is more difficult. At present, the growth rate of SiC crystals is slow, and it is very easy to produce defects in the production process, resulting in low device yield. In addition, the high hardness of SiC makes it difficult to process, further limiting its large-scale application.
3. Device reliability: Although SiC performs well in extreme environments such as high temperatures and high pressures, the long-term reliability of some SiC devices still needs to be further improved in practical applications. Compared with the mature silicon process, the aging problem of SiC devices under extreme conditions has not yet been fully resolved, and the demand for longer life cycles in some application scenarios requires further enhancement of their device stability and reliability.
6 Conclusion
In conclusion, silicon carbide (SiC) has established itself as a crucial material in electronic materials due to its unique advantages, including high thermal conductivity, hardness, and superior performance in high-temperature, high-pressure, and high-frequency environments. Its application spans multiple sectors—power electronics, high-temperature and high-frequency devices, LEDs, sensors, and aerospace—which benefit from SiC’s efficiency in energy conversion, extended device life, and stability in extreme conditions. However, high costs, complex fabrication processes, and reliability concerns still limit SiC’s widespread adoption. As advances in manufacturing technologies and cost-efficiency continue, silicon carbide is expected to play an even more prominent role in high-performance and specialized electronic applications.
Stanford Advanced Materials (SAM) is a key provider of high-quality silicon carbide materials, supporting these critical applications with reliable material solutions.
Related Reading
The Impact of Silicon Wafer Quality on Semiconductor Performance and Reliability
Comparing SOI vs. Silicon Wafers: What’s Best for Your Semiconductor Project?
Silicon Carbide vs. Silicon: A Comparative Study of Semiconductors in High-Temperature Applications
Case Study: Silicon Carbide Plates for Advanced Armor Solutions
The Breakthrough of Silicon Carbide Substrate in LED Industry