A Comprehensive Guide to Amorphous Metals
1. Introduction to Amorphous Metals
Amorphous metals, also known as metallic glasses, are a unique class of materials characterized by their disordered atomic structure. Unlike crystalline metals, which have a regular, repeating atomic arrangement, amorphous metals lack this order, resulting in distinct properties. This lack of crystallinity imparts a combination of high strength, elasticity, and corrosion resistance, making these materials highly desirable for various advanced applications.
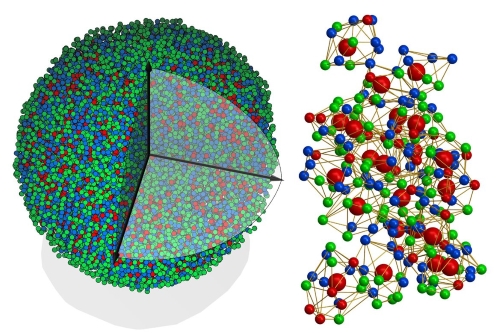 [1]
[1]
2. Production Methods
Amorphous metals are typically produced through rapid cooling processes, which prevent the atoms from arranging into a crystalline structure. Common methods include:
- Melt Spinning: Molten metal is rapidly cooled on a rotating wheel, forming thin ribbons. This method is widely used in producing amorphous metal tapes for transformers and other magnetic applications.
- Splat Quenching: A droplet of molten metal is rapidly cooled between two cold surfaces, forming thin, flat disks of amorphous metal. This method is used in laboratory settings for quick material analysis and small-scale production.
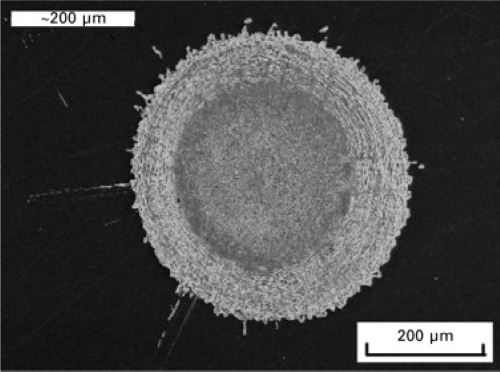 [2]
[2]
- PVD (Physical Vapor Deposition): Metal atoms are deposited on a substrate in a vacuum environment, allowing for the controlled formation of amorphous films. This technique is commonly used in the electronics industry for creating thin films with specific magnetic or optical properties.
3. Properties and Applications
Amorphous metals come with unique properties, including high strength, elasticity, and corrosion resistance:
- High Strength: Amorphous metals often have higher tensile strength compared to their crystalline counterparts due to the absence of grain boundaries. For example, metallic glass alloys like Vitreloy 1 exhibit a tensile strength of up to 1.9 GPa, significantly higher than that of traditional steel.
- Elasticity: These metals can exhibit significant elastic strain, making them highly resilient. Amorphous metals can undergo elastic strain up to 2% compared to crystalline metals, which typically show around 0.2% elastic strain.
- Corrosion Resistance: The lack of grain boundaries and homogenous structure leads to excellent resistance to corrosion. For instance, Zr-based metallic glasses have shown superior corrosion resistance in saline environments, making them ideal for marine applications.
- Magnetic Properties: Certain amorphous metals exhibit soft magnetic properties, making them useful in transformer cores and magnetic shielding. For example, amorphous iron-based alloys have lower coercivity and core loss than crystalline iron, improving energy efficiency in transformers.
- Electrical Resistance: High electrical resistance is another notable feature, which can be beneficial in specific applications such as resistors and magnetic sensors.
Amorphous metals find applications in various industries due to their unique properties:
- Electronics: Used in transformer cores and inductors, particularly in high-frequency applications where low energy loss is critical. For instance, amorphous metal cores can reduce energy losses by up to 70% compared to traditional silicon steel cores.
- Biomedical Devices: Their biocompatibility and corrosion resistance make them suitable for medical implants and surgical tools. Zr-based metallic glasses are particularly used in stents and orthopedic implants.
- Sporting Goods: Used in high-performance sporting equipment, such as golf clubs and tennis rackets, for their strength and elasticity. The elasticity of metallic glass helps in better energy transfer, enhancing the performance of the equipment.
- Defense and Aerospace: Employed in lightweight armor and structural components that require high strength-to-weight ratios. Amorphous metal coatings are also used to protect aerospace components from wear and corrosion.
- Consumer Electronics: Used in casings and structural components due to their durability and scratch resistance. The Apple Watch, for example, uses a metallic glass alloy for its case due to its strength and smooth finish.
4. Challenges and Developments
Yet, amorphous metals face several challenges that have limited their widespread adoption and applications. Among the primary obstacles are production costs, size limitations, and brittleness, each posing significant hurdles in different contexts.
The foremost challenge is the high production cost. The process of rapidly cooling molten metal to prevent crystallization requires specialized equipment and precise control, making the manufacturing process both complex and expensive. This need for rapid cooling often necessitates the use of advanced, high-cost machinery, which limits the ability to produce amorphous metals at scale. Consequently, their use has been largely restricted to high-value applications where the benefits outweigh the production expenses.
Another significant limitation is the difficulty in producing large, bulk amorphous metal components. The rapid cooling essential to maintaining the amorphous structure becomes increasingly challenging as the size of the component increases. As a result, most amorphous metals are currently available only in small forms such as ribbons, wires, or thin sheets. This limitation has restricted their application to smaller items and niche markets.
Also, brittleness remains a critical concern, particularly in structural applications where materials are expected to withstand significant stress and strain. Although amorphous metals are renowned for their strength, the absence of a crystalline structure can lead to brittleness, making them prone to fracture under certain conditions. This brittleness is particularly problematic in applications requiring materials that can absorb impact or undergo deformation without breaking.
In response to these challenges, significant advancements have been made in the field of amorphous metals:
- Bulk Metallic Glasses (BMGs): Developing larger amorphous metal components for industrial use. For example, BMGs have been developed with improved ductility, making them more suitable for structural applications in automotive and aerospace industries.
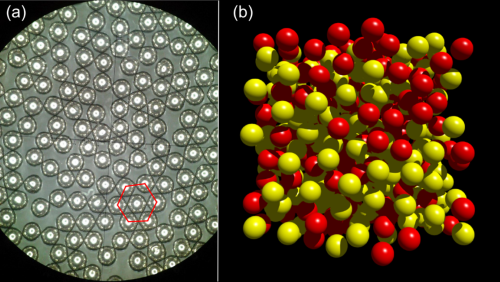 [3]
[3]
- Advanced Alloys: Creating new compositions that enhance the properties of amorphous metals, such as improved ductility or higher corrosion resistance. Pd-based and Cu-based metallic glasses are notable for their enhanced mechanical properties.
- Additive Manufacturing: Exploring the use of 3D printing techniques to produce complex amorphous metal structures. This approach could revolutionize the production of customized components with superior properties, such as dental implants and complex aerospace parts.
5. Amorphous Metals vs. Metallic Glasses
The terms "amorphous metals" and "metallic glasses" are often used interchangeably. They refer to the same class of materials. However, there are subtle distinctions in how these terms are used, which can be important to understand.
--Amorphous Metals
Amorphous metals are metals with a disordered atomic structure, lacking the regular, repeating pattern found in crystalline metals. This disordered structure is achieved by rapidly cooling the molten metal, preventing the atoms from arranging into a crystalline lattice.
The term "amorphous metal" emphasizes the metal's atomic disorder and is often used when discussing the broader category, including various manufacturing methods and applications.
--Metallic Glasses
Metallic glasses are a subset of amorphous metals that specifically exhibit a glass-like structure. This term highlights the material's non-crystalline, "glassy" state, which is similar to that of conventional glasses like silica glass, but made from metallic alloys.
The term "metallic glass" is frequently used in scientific and academic contexts, particularly when discussing the physical and mechanical properties related to the glassy state, such as brittleness and elastic behavior.
In summary, while "amorphous metals" and "metallic glasses" refer to the same general type of material, the former term is broader and more commonly used in industrial contexts, whereas the latter is more specific and often used in scientific research to describe the glassy characteristics of these materials. Understanding these distinctions can help in accurately communicating the material's properties and potential applications.
6. Conclusion
Amorphous metals, with their unique disordered atomic structure, represent a significant advancement in material science. Their combination of high strength, elasticity, and corrosion resistance sets them apart from traditional crystalline metals, making them indispensable in electronics, biomedical devices, defense, and aerospace.
Despite the challenges posed by high production costs, size limitations, and brittleness, ongoing research and innovation continue to push the boundaries of what is possible with these remarkable materials. As industries seek materials that can meet the demands of modern technology and innovation, amorphous metals are poised to shape the future of high-performance applications. For more information, please check Stanford Advanced Materials (SAM).
Reference:
[1] UCLA News (2021, March 31). Century-old problem solved with first-ever 3D atomic imaging of an amorphous solid. Retrieved August 20, 2024, from https://newsroom.ucla.edu/releases/first-ever-3d-atomic-imaging-amorphous-solid
[2] Y.C. Xin, P.K. Chu, 11 - Plasma immersion ion implantation (PIII) of light alloys, Editor(s): Hanshan Dong, In Woodhead Publishing Series in Metals and Surface Engineering, Surface Engineering of Light Alloys, Woodhead Publishing, 2010, Pages 362-397, https://www.sciencedirect.com/science/article/pii/B9781845695378500117
[3] University of Vienna (2024, August 20). Structural inhomogeneities in bulk metallic glasses. Retrieved August 20, 2024, from https://sounds-of-matter.univie.ac.at/research-projects/metallic-glass/



