Substrates, Supports, and Ligands in Precious Metal Catalysts
Introduction
Precious metal catalysts are widely used in various industrial applications due to their exceptional catalytic properties. However, their performance is significantly influenced by the materials to which they are attached—referred to as substrates, supports, or ligands. These materials play a crucial role in determining the catalyst's activity, stability, selectivity, and regeneration ability. Here's a detailed overview of these components and their importance in catalytic reactions.
1. Substrate: The Foundation of Catalytic Reactions
A substrate serves as the surface material on which precious metals are attached or dispersed during catalytic reactions. This is particularly relevant for heterogeneous catalysts. The choice of substrate is critical as it affects the dispersion, surface area, and catalytic activity of the precious metals.
Common substrate materials include:
- Alumina (Al₂O₃): Alumina is known for its large surface area and good mechanical strength, making it a popular choice for substrates in hydrogenation, oxidation, and reforming reactions.
- Silica (SiO₂): Silica substrates are chemically inert and highly thermally stable, making them suitable for catalytic processes requiring high selectivity.
- Carbon Materials: Activated carbon and carbon nanotubes offer excellent electrical conductivity and a large surface area, making them ideal substrates for fuel cell catalysts and certain reduction reactions.
2. Support: Enhancing Catalyst Performance
 [1]
[1]
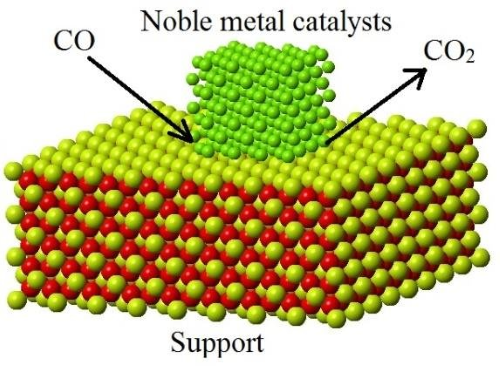
Supports are materials used to disperse precious metals on the catalyst's surface, commonly used in heterogeneous catalysts. The primary function of supports is to provide a high surface area to support the dispersion of precious metals while stabilizing the catalyst's activity.
--Porous Oxides
Porous oxides like alumina, silica, and titania are valued for their high surface area and stability, making them ideal for dispersing precious metals.
- Alumina (Al₂O₃): Widely used for its large surface area (100-300 m²/g) and stability in high-temperature processes like hydrogenation and reforming.
- Silica (SiO₂): Chosen for its inertness and thermal stability (200-600 m²/g), suitable for oxidation reactions and catalytic cracking.
- Titania (TiO₂): Known for its photocatalytic properties, used in light-activated processes and emissions control in automotive applications.
--Carbon Supports
Carbon supports, including carbon black and activated carbon, are essential in electrochemical applications due to their conductivity and large surface area.
- Carbon Black: Provides excellent conductivity, commonly used in fuel cells, where platinum on carbon black (Pt/C) plays a key role in oxygen reduction.
- Activated Carbon: With an exceptionally high surface area (500-1500 m²/g), it’s ideal for adsorption and filtration processes, effectively supporting reactions like hydrogenation.
--Metal Oxides
Metal oxides like ceria and zirconia offer unique redox properties, enhancing the interaction with precious metals and boosting catalytic efficiency.
- Ceria (CeO₂): Effective in oxidation-reduction reactions, particularly in automotive catalytic converters, due to its oxygen storage capacity.
- Zirconia (ZrO₂): Known for its thermal stability and robustness in harsh conditions, it’s commonly used in high-temperature isomerization processes.
3. Ligands: Tuning Catalytic Properties
Ligands are molecules or ions that form coordination bonds with the precious metal center, predominantly used in homogeneous catalysts. The structure and properties of ligands directly impact the catalyst's activity, selectivity, and stability.
Here are common ligand types:
- Phosphine Ligands: Compounds like triphenylphosphine (PPh₃) are widely used in palladium-catalyzed cross-coupling reactions, where they regulate the reaction's selectivity and rate.
- Nitrogen-Based Ligands: Ligands such as pyridine and bipyridine can adjust the electronic density of precious metals, influencing the catalytic reaction's activity and selectivity.
- Chelating Ligands: Ligands like EDTA can form stable chelates with precious metals, enhancing the catalyst's stability, particularly in complex organic reactions.
Factors Influencing Catalyst Performance
The performance of precious metal catalysts is determined by several factors associated with substrates, supports, and ligands.
- Surface Area and Porosity: The surface area and porosity of substrates and supports directly impact the dispersion of precious metals and the availability of active sites.
- Chemical Stability: The chemical stability of supports and ligands determines the catalyst's durability in extreme environments, such as high temperatures or strong acidic/alkaline conditions.
- Electronic Effects and Coordination Environment: The electronic properties and coordination environment provided by ligands can greatly affect the reaction pathways and selectivity of the catalyst.
Tailoring Catalysts for Specific Industrial Applications
The choice of substrate, support, and ligand combinations is often driven by the specific requirements of different industrial applications. These materials must be carefully selected to match the reaction conditions and desired outcomes.
For instance:
- Hydrogenation Reactions: Alumina-supported catalysts are widely used in hydrogenation due to their high surface area and mechanical strength.
- Fuel Cells: Carbon-supported precious metal catalysts are essential in fuel cells, where high conductivity and chemical stability are required.
- Pharmaceutical Synthesis: Ligand-modified catalysts are often employed in pharmaceutical synthesis to achieve high selectivity and efficiency in complex organic reactions.
Application | Catalyst Component | Key Materials |
Hydrogenation Reactions | Substrate | Alumina (Al₂O₃) |
Substrate | Silica (SiO₂) | |
Fuel Cells | Support | Carbon Black (Pt/C) |
Support | Graphene | |
Pharmaceutical Synthesis | Ligand | Phosphine-modified Palladium (Pd/PPh₃) |
Ligand | Chiral Ligands (e.g., BINAP) | |
Oxidation Reactions | Support | Ceria (CeO₂) |
Support | Titania (TiO₂) | |
Reforming and | Support | Zirconia (ZrO₂) |
Substrate | Alumina (Al₂O₃) | |
Polymerization | Support | Ziegler-Natta (TiCl₄/MgCl₂) |
Support | Metallocene (Silica/Alumina supported) |
For more cases and examples, please check Stanford Advanced Materials (SAM).
Related reading: Common Reaction Types of Homogeneous Precious Metal Catalysts
Conclusion:
The selection of appropriate substrates, supports, and ligands is crucial for optimizing the performance of precious metal catalysts. By carefully choosing these materials, it is possible to tailor the catalyst's properties to meet the specific requirements of different industrial applications, thereby enhancing efficiency and extending the catalyst's lifespan.
Reference:
[1] Hossain, Shaikh. (2018). Synthesis and Kinetic Study of CeO2 and SiO2 Supported CuO Catalysts for CO Oxidation. 10.13140/RG.2.2.31499.80165.