Common Reaction Types of Homogeneous Precious Metal Catalysts
Introduction
Precious metals such as platinum, palladium, rhodium, and gold are commonly used as homogeneous catalysts due to their high catalytic activity, selectivity, and stability. They are also known for their great thermal stability and chemical inertness, which makes them exceptional catalysts. With these characteristics, homogeneous precious metal catalysts find a wide range of applications, such as pharmaceuticals, petrochemicals, chemicals, and material sciences.
In this article, we will talk about the common reaction types of homogeneous precious metal catalysts. Hope that you can have a further comprehension of these valuable catalysts.

Figure 1. Precious Metal Catalysts
Common Reaction Types of Homogeneous Precious Metal Catalysts
Homogeneous precious metal catalysts are utilized in a variety of reactions. Typical examples are hydrogenation, hydroformylation reactions, coupling reactions, etc.
--Hydrogenation:
Hydrogenation is a reaction in which hydrogen is added to unsaturated organic compounds, typically with the help of a catalyst. Homogeneous catalysts such as platinum and palladium are extensively used in hydrogenation reactions to convert alkenes to alkanes, and nitro compounds to amines.

Figure 2. Metal-catalyzed hydrogenation and dehydrogenation reactions for efficient hydrogen storage [1]
--Dehydrogenation:
Dehydrogenation is the opposite of hydrogenation, in which hydrogen is removed from a molecule. Precious metal catalysts such as platinum and rhodium are employed in dehydrogenation reactions to make alkenes from alkanes, and carbonyl compounds from alcohols.
--Oxidation:
In oxidation reactions, a molecule loses electrons, and homogeneous precious metal catalysts are used to convert alcohols to aldehydes or ketones, and alkenes to epoxides. Among these oxidation reactions, the "Hoechst-Wacker" process is the most famous one, in which acetaldehyde is synthesized from ethene and oxygen using Pd/Cu catalysts in aqueous, chloride-containing solutions.
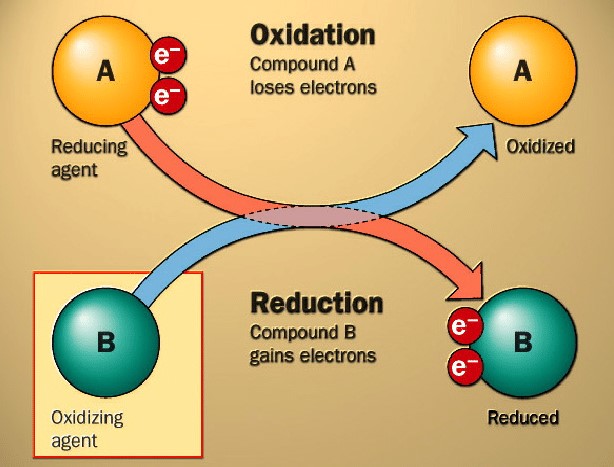
Figure 3. Basic oxidation and reduction [2]
--Reduction:
Reduction is the opposite of oxidation, in which a molecule gains electrons. These homogeneous catalysts are generally utilized in reduction reactions to convert nitro compounds to amines, and carbonyl compounds to alcohols.
--Coupling:
Coupling reactions involve the joining of two or more molecules to form a larger molecule. Palladium, platinum, and other catalysts are applied to coupling reactions to form carbon-carbon bonds, such as in the Suzuki reaction and the Heck reaction.
--Carbonylation:
Carbonylation refers to reactions that form aldehydes, ketones, etc. using carbon monoxide (CO). The best-known process is the carbonylation of methanol to acetic acid. It is also called Monsanto Process. All these processes cannot be achieved without rhodium catalysts.
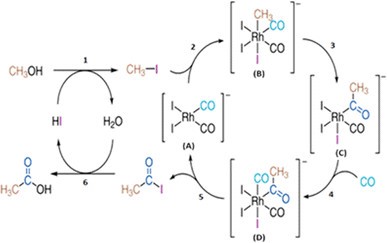
Figure 4. Proposed catalytic cycles of the rhodium-catalyzed methanol carbonylation reaction (Monsanto process) [3]
--Hydroformylation:
Hydroformylation is also known as oxo synthesis. This process converts alkenes into aldehydes with a mixture of carbon monoxide (CO) and hydrogen (H2). Rhodium catalysts took the place of the former cobalt catalyst in such processes.
--Isomerization:
Isomerization is a reaction in which a molecule undergoes a structural rearrangement. Platinum and rhodium are typical homogeneous catalysts used in isomerization reactions to convert alkanes to branched alkanes, and alkenes to isomers.
You can check the table below to learn more about the comparison between different reaction types of homogeneous precious metal catalysts.
Table 1. Different Reaction Types of Homogeneous Precious Metal Catalysts
| Definition | Examples |
Hydrogenation | Adding hydrogen; | Converting alkenes to alkanes, and nitro compounds to amines; |
Dehydrogenation | Removing hydrogen; | Converting alkanes to alkenes, and alcohols to carbonyl compounds; |
Oxidation | Losing elections; | Converting alcohols to aldehydes or ketones, and alkenes to epoxides; |
Reduction | Gaining electrons; | Converting nitro compounds to amines, and carbonyl compounds to alcohols; |
Coupling | Joining of two or more molecules to form a larger molecule; | The Suzuki reaction and the Heck reaction; |
Carbonylation | Forming aldehydes and ketones using carbon monoxide (CO); | The Monsanto Process; |
Hydroformylation | Converting alkenes into aldehydes with carbon monoxide (CO) and hydrogen (H2); | Using rhodium catalysts; |
Isomerization | Structural Arrangements; | Convert alkanes to branched alkanes, and alkenes to isomers; |
Conclusion
In a word, homogeneous precious metal catalysts are widely used in various chemical reactions, including hydrogenation, dehydrogenation, oxidation, reduction, coupling, carbonylation, hydroformylation, and isomerization. Their high catalytic activity, selectivity, and stability make them invaluable tools for chemists in the pharmaceutical, petrochemical, and fine chemical industries. By understanding the common reaction types of homogeneous precious metal catalysts, scientists can develop more efficient and sustainable chemical processes.
Stanford Advanced Materials (SAM) is a reliable supplier of platinum catalysts, palladium catalysts, and other precious metal catalysts. Send us an inquiry if you are interested.
Reference:
[1] Shimbayashi, Takuya & Fujita, Ken-Ichi. (2020). Metal-catalyzed hydrogenation and dehydrogenation reactions for efficient hydrogen storage. Tetrahedron. 76. 130946. 10.1016/j.tet.2020.130946.
[2] Azman, Nur & Ramli, Muhammad & Isa, Siti. (2019). A Review of hybridization of carbon nanotube into graphene for gas sensor application. IOP Conference Series: Materials Science and Engineering. 551. 012017. 10.1088/1757-899X/551/1/012017.
[3] Budiman, Anatta & Nam, Ji & Park, Jae & Mukti, Ryan & Chang, Tae & Bae, Jong Wook & Choi, Myoung. (2016). Review of Acetic Acid Synthesis from Various Feedstocks Through Different Catalytic Processes. Catalysis Surveys from Asia. 20. 10.1007/s10563-016-9215-9.