Titanium Dioxide Polymorphs: Rutile vs. Anatase
Introduction
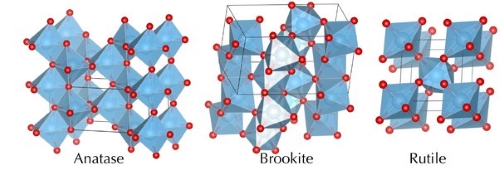
Titanium dioxide (TiO₂) is a widely utilized compound in various industries, appreciated for its unique optical, physical, and chemical properties. It naturally occurs in three polymorphic forms: anatase, rutile, and brookite. Among these, anatase and rutile are the most significant for industrial applications, while brookite is rarely used due to its instability. This article will discuss the key differences between anatase and rutile, highlighting their structures, properties, and applications.
 [1]
[1]
Crystal Structure and Stability
Both anatase and rutile belong to the tetragonal crystal system but differ in their lattice structures and stability.
- Anatase: This polymorph has a more open crystal structure, with a unit cell containing four TiO₂ molecules. Its crystal lattice is less compact, resulting in lower density. Anatase is stable at room temperature but transforms into a more stable phase at elevated temperatures, typically around 730°C. This transformation is irreversible and exothermic, highlighting the higher thermodynamic stability of the final phase.
- Rutile: Characterized by a denser and more compact crystal structure, each unit cell contains two TiO₂ molecules. This close-packed arrangement results in a higher density and greater stability. It is the most thermodynamically stable form of TiO₂, and both anatase and brookite convert into this stable phase upon heating.
Physical Properties
--Density and Hardness
The relative density of anatase ranges from 3.8 to 3.9 g/cm³, and its Mohs hardness is between 5.5 and 6.0. The lower density and hardness make anatase less durable than rutile.
With a relative density of 4.2 to 4.3 g/cm³, rutile is denser and more compact. Its Mohs hardness ranges from 6.0 to 7.0, making it more suitable for applications requiring higher durability and wear resistance.
--Dielectric Constant
The dielectric constant of anatase is around 48, which is significantly lower than that of rutile. This lower dielectric constant limits its use in applications that require high dielectric properties.
Rutile has a much higher dielectric constant, averaging around 114. This high dielectric constant, coupled with its stability, makes it ideal for electronic applications.
Optical Properties
--Refractive Index
The refractive index of a material determines its ability to bend light, and TiO₂ is known for having a very high refractive index, which is beneficial for optical applications. The refractive index of anatase is approximately 2.55. While high, it is still lower than that of rutile.
Rutile boasts an even higher refractive index, around 2.71, making it exceptionally effective in applications requiring maximum light scattering and opacity.
--Scattering Power
The light scattering ability of TiO₂ is crucial for its use as a pigment in paints, coatings, and other materials. Despite having good light scattering properties, anatase's lower refractive index means it is less effective than rutile.
With its higher refractive index, rutile provides superior light scattering, enhancing opacity and brightness in applications like paints and coatings. This makes it the preferred choice for white pigments.
Electrical Properties
--Conductivity
Titanium dioxide acts as a semiconductor, with its electrical conductivity influenced by temperature and oxygen vacancies. Generally, anatase exhibits lower electrical conductivity. It is less sensitive to temperature changes compared to rutile.
Rutile’s electrical conductivity increases significantly with temperature. At around 420°C, its conductivity can increase by several orders of magnitude, making it valuable in electronic components such as ceramic capacitors. This sensitivity to temperature and oxygen content makes it useful in sensing applications.
Applications
Both anatase and rutile have distinct applications based on their respective properties.
1. Anatase
- Photocatalysis: Anatase is widely used in photocatalytic applications due to its higher reactivity under UV light. It is effective in degrading organic pollutants, making it useful for air and water purification systems, self-cleaning surfaces, and antimicrobial coatings.
- Solar Cells: Due to its photoactive properties, anatase is employed in dye-sensitized solar cells to enhance efficiency.
2. Rutile
- Pigments: Its high refractive index and superior light scattering make it ideal for use as a white pigment in paints, plastics, and papers. It provides excellent opacity and brightness.
- Optical Components: It is used in the production of optical components, such as lenses and coatings, due to its high refractive index.
- Electronics: Its high dielectric constant and electrical conductivity under high temperatures make rutile suitable for electronic devices, including capacitors and varistors.
- High-Temperature Applications: Its stability at high temperatures makes it suitable for ceramic glazes, refractory materials, and other high-temperature applications.
Quick Facts about Rutile and Anatase
|
Property |
||
|
Density (g/cm3) |
3.8 - 3.9 |
4.2 - 4.3 |
|
Mohs Hardness |
5.5 - 6.0 |
6.0 - 7.0 |
|
Dielectric Constant |
48 |
114 |
|
Refractive Index |
2.55 |
2.71 |
|
Scattering Power |
Good |
Superior |
|
Electrical Conductivity |
Lower, less sensitive to temperature changes |
Higher, increases with temperature |
|
Common Applications |
Photocatalysts, solar cells, paper, inks, textiles, rubber, ceramics, cosmetics |
Coatings, air purification, military applications, cosmetics, paints, and plastic items |
Stanford Advanced Materials (SAM) offers premium Titanium products at competitive prices. We supply both Anatase and Rutile forms of Titanium Dioxide, with customization options available to meet your specific needs. Contact us for more information or to place an inquiry.
Conclusion
Understanding the differences between anatase and rutile is essential for optimizing their use in various industrial applications. Anatase, with its higher photocatalytic activity, is suitable for environmental and self-cleaning technologies. Rutile, on the other hand, offers superior stability, density, and optical properties, making it ideal for pigments, coatings, and electronic components.
The choice between them depends on the specific requirements of the application. By leveraging the unique properties of these TiO₂ polymorphs, industries can enhance the performance and efficiency of their products.
Reference:
[1] Stawarz, Sylwester & Witek, Natalia & Kucharczyk, Wojciech & Bakar, Med & Stawarz, Magdalena. (2019). Thermo-protective properties of polymer composites with nano-titanium dioxide. International Journal of Mechanics and Materials in Design. 15. 10.1007/s10999-018-9432-7.