6 Common Coating Methods for Non-Ferrous Metals
Introduction
Non-ferrous metals, including aluminum, copper, zinc, and titanium, are prized for their unique properties such as low weight, high conductivity, and excellent corrosion resistance. To further enhance these properties and extend the lifespan of non-ferrous metals, various types of coatings are applied. These coatings provide additional protection against environmental factors, improve aesthetic appeal, and enhance surface properties. In this guide, we will explore the common types of coatings used for non-ferrous metals and their benefits, with specific case examples.
1. Anodizing
Anodizing is an electrochemical process predominantly used for aluminum and its alloys. During anodizing, the metal surface is converted into a durable, corrosion-resistant anodic oxide finish. This process involves immersing the aluminum in an electrolytic solution, where it acts as an anode. When an electric current is passed through the solution, oxygen ions are released from the electrolyte and combine with the aluminum atoms on the surface, forming a thick oxide layer.
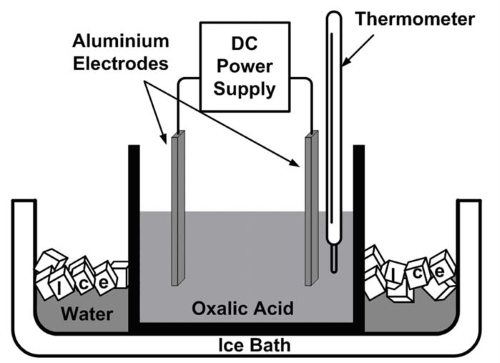 [1]
[1]
Benefits of Anodizing:
- Corrosion Resistance: The anodic oxide layer is highly resistant to corrosion, making it ideal for outdoor applications and harsh environments.
- Surface Hardness: Anodizing significantly increases the surface hardness of aluminum, improving its wear resistance.
- Aesthetic Appeal: The porous nature of the anodized layer allows for coloring through dyeing, offering a wide range of aesthetic options.
- Thermal Insulation: The anodized layer provides thermal insulation, making it suitable for applications requiring heat resistance.
For example, in consumer electronics, anodizing is commonly used for aluminum housings in smartphones, laptops, and tablets. The process not only provides a sleek and durable finish but also enhances scratch resistance, ensuring the devices maintain their aesthetic appeal over time.
2. Electroplating
Electroplating involves depositing a thin layer of metal onto the surface of another metal through an electrochemical process. Common metals used for electroplating non-ferrous metals include nickel, chromium, copper, and gold. The process entails immersing the metal (cathode) and a plating metal (anode) in an electrolytic solution. An electric current is then applied, causing the plating metal to dissolve and deposit onto the cathode.
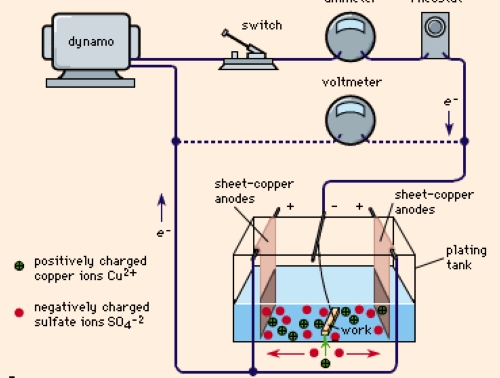
Benefits of Electroplating:
- Enhanced Appearance: Electroplating can provide a shiny, attractive finish, improving the metal's aesthetic appeal.
- Corrosion Resistance: The plated layer offers additional protection against corrosion and oxidation.
- Improved Surface Properties: Electroplating can enhance surface conductivity, hardness, and wear resistance, making it suitable for electrical components and decorative items.
- Reduced Friction: Certain electroplating materials can reduce friction, which is beneficial for mechanical components.
For instance, gold and silver electroplating are extensively used in the jewelry industry to provide a luxurious finish to base metals such as copper and brass. This process allows for affordable yet high-quality pieces that retain their luster and resist tarnishing.
3. Powder Coating
Powder coating is a dry finishing process widely used for aluminum, magnesium, and other non-ferrous metals. It involves applying a dry powder, typically composed of thermoplastic or thermoset polymer, electrostatically onto the metal surface. The coated metal is then cured under heat, causing the powder to melt and form a hard, uniform finish.
Benefits of Powder Coating:
- Durability: Powder coating provides a thick, robust finish that is highly resistant to chipping, scratching, and fading.
- Corrosion Resistance: The coating offers excellent protection against corrosion, making it suitable for outdoor and marine environments.
- Environmental Friendliness: Powder coating produces minimal volatile organic compounds (VOCs) compared to traditional liquid coatings.
- Variety of Finishes: Available in a wide range of colors and textures, powder coating offers versatility in design and aesthetics.
In terms of automotive parts, powder coating is commonly used for automotive parts such as wheels, frames, and suspension components. The coating provides a durable and aesthetically pleasing finish that withstands the harsh conditions of the road.
4. CVD & PVD
Chemical Vapor Deposition (CVD) and Physical Vapor Deposition (PVD) are advanced coating processes used to apply thin films of various materials onto non-ferrous metals. CVD involves chemical reactions that occur in a vapor phase, while PVD involves the physical deposition of material from a vapor phase.
Benefits of CVD & PVD:
- Superior Coating Quality: Both processes provide high-quality, uniform coatings with excellent adhesion.
- Enhanced Surface Properties: These coatings can significantly improve hardness, wear resistance, and corrosion resistance.
- Versatility: CVD and PVD can be used to deposit a wide range of materials, including metals, ceramics, and polymers.
- High Precision: These processes offer precise control over coating thickness and composition, making them ideal for specialized applications.
For example, in the aerospace industry, CVD and PVD are used to coat turbine blades and other components to enhance their performance and durability under extreme conditions.
5. Passivation
Passivation is a chemical treatment process used to enhance the corrosion resistance of stainless steel, titanium, and other alloys. The process involves removing free iron from the metal surface and promoting the formation of a thin, inert oxide layer. This oxide layer acts as a barrier, preventing further oxidation and corrosion.
Benefits of Passivation:
- Improved Corrosion Resistance: The passivated layer significantly enhances the metal's resistance to corrosion.
- Surface Cleanliness: Passivation removes contaminants from the surface, ensuring a clean and passive finish.
- Aesthetic Maintenance: The process helps maintain the metal's natural appearance and prevents tarnishing.
- Longevity: By preventing corrosion, passivation extends the lifespan of the metal components.
For instance, passivation is critical for stainless steel medical devices such as surgical instruments and implants. The process ensures that the devices remain free from corrosion and maintain their biocompatibility.
6. Organic Coatings
Organic coatings include paints, varnishes, and polymers applied to non-ferrous metals to provide protection and decorative finishes. These coatings form a protective barrier that shields the metal from environmental factors such as moisture, chemicals, and UV radiation.
Benefits of Organic Coatings:
- Versatility: Organic coatings are available in various formulations, colors, and finishes, offering flexibility in design.
- Corrosion Resistance: They provide an effective barrier against corrosion and environmental degradation.
- Ease of Application: Organic coatings can be easily applied through spraying, brushing, or dipping.
- Cost-Effective: These coatings are relatively inexpensive and provide a quick and effective solution for protecting non-ferrous metals.
For example, organic coatings are extensively used in architectural applications, including window frames, doors, and facades. These coatings enhance the appearance of buildings while protecting the underlying metal from weathering and corrosion.
Conclusion
The choice of coating for non-ferrous metals depends on the specific requirements, including the desired level of corrosion resistance, aesthetic appeal, and surface properties. By selecting the appropriate coating, manufacturers can significantly enhance the performance, durability, and longevity of non-ferrous metal components, ensuring they meet the demands of various industries and environments. Hope that you can find the perfect coated products at Stanford Advanced Materials (SAM).
Reference:
[1] Ahmad, Hafiz Imran & Sharif, Muhammad & Hussain, Safdar & Badar, M. & Afzal, H.. (2013). Spectroscopic Study of a Radio-Frequency Atmospheric Pressure Dielectric Barrier Discharge with Anodic Alumina as the Dielectric. Plasma Science and Technology. 15. 900. 10.1088/1009-0630/15/9/13.
[2] Electroplating. (2024, May 16). In Britannica. https://www.britannica.com/technology/electroplating