Discovery of the Elements
Introduction
The discovery of the elements is a fascinating journey through the history of science and human understanding of the natural world. Elements, the fundamental substances that makeup matter, have been a subject of curiosity and exploration for centuries. This article delves into the evolution of our knowledge about elements, from ancient times to the modern era, highlighting key discoveries and the individuals who played pivotal roles in uncovering the building blocks of the universe.
Ancient Insights
The earliest records of elemental exploration can be traced back to ancient civilizations. Ancient Greek philosophers such as Empedocles proposed the idea of four basic elements: Earth, Water, Air, and Fire. This early theory, known as the "classical elements," influenced scientific thought for centuries.
Alchemical Pursuits
During the Middle Ages and the Renaissance, alchemy was a prevalent practice. Alchemists sought not only to transform base metals into noble ones but also to discover the elusive Philosopher's Stone and Elixir of Life. While these pursuits were often shrouded in mysticism, they laid the groundwork for the systematic study of chemical processes, ultimately leading to the discovery of new elements.
The Age of Enlightenment
The 18th century marked a significant turning point in the understanding of elements. Antoine Lavoisier, often referred to as the "Father of Modern Chemistry," conducted pioneering experiments that challenged the classical elements theory. He formulated the Law of Conservation of Mass and introduced the concept of chemical elements as substances that could not be broken down further by chemical means.
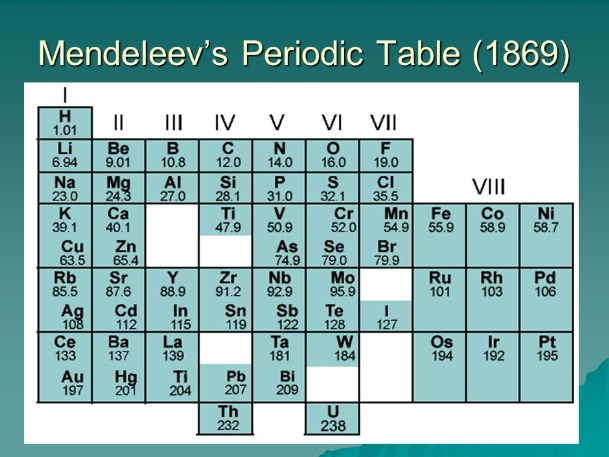
The Periodic Table
The 19th century witnessed remarkable progress in the discovery of elements, thanks in large part to Dmitri Mendeleev's development of the periodic table. Mendeleev's table, arranged by atomic weight and chemical properties, allowed for the prediction of undiscovered elements and their properties. His predictions, such as the discovery of gallium and germanium, provided substantial evidence for the validity of his periodic table.
Synthesis of Synthetic Elements
As the periodic table expanded, some elements could not be found in nature and had to be synthesized in laboratories. Glenn T. Seaborg, an American chemist, is renowned for his work in creating transuranium elements, such as americium and curium. His groundbreaking research expanded the periodic table to include elements beyond uranium.
The Birth of Modern Chemistry
The 20th century brought unprecedented advancements in the discovery of elements. The advent of nuclear physics and particle accelerators facilitated the creation and identification of synthetic elements. Prominent among these is Glenn T. Seaborg, who was instrumental in the synthesis of several transuranium elements, expanding the periodic table beyond uranium.
In contemporary times, scientists continue to pursue the discovery of new elements, often with the aid of advanced particle accelerators and nuclear reactions. Elements 113 (Nihonium, Nh), 114 (Flerovium, Fl), and 118 (Oganesson, Og) are among the most recently discovered elements, with their existence confirmed in the 21st century.
The Standard Model of Particle Physics
In the realm of particle physics, the search for elements extends to subatomic particles. The discovery of quarks and their role in forming protons, neutrons, and atomic nuclei deepened our understanding of the fundamental constituents of matter.
Conclusion
The discovery of the elements is a testament to human curiosity, innovation, and the relentless pursuit of knowledge. From the ancient Greeks to modern scientists, the quest to unravel the mysteries of matter has reshaped our understanding of the universe. The periodic table, an enduring symbol of this journey, continues to evolve as new elements are synthesized and added to the ever-expanding catalog of elements. The discovery of the elements remains a cornerstone of scientific exploration, enriching our understanding of the natural world and shaping the course of chemistry and physics.