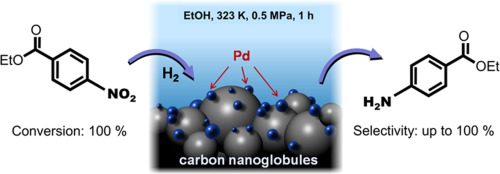
High-Speed Steel vs Tungsten Steel
High-speed steel vs tungsten steel, what's the difference? If you want to find the answer to this question, then you've come to the right place. In this article, we will take a closer look at the differences between high-speed steel and tungsten steel.

High-Speed Steel vs Tungsten Steel
What is high-speed steel?
High-Speed Steel (HSS) is a tool steel with high hardness, wear resistance, and heat resistance. HSS is a kind of complex alloy steel, which contains carbide-forming elements such as tungsten, molybdenum, chromium, vanadium, and cobalt, and the total amount of alloy elements is about 10-25%.
HSS is mainly used to manufacture complex thin blade and impact-resistant metal cutting tools, as well as high-temperature bearing and cold extrusion die, such as a turning tool, drill bit, hob, machine saw blade, high demand die, etc. It can still maintain a high hardness in high speed cutting under the condition of high temperature (500 ℃), which is the main characteristic of high-speed steel, the red hardness.
After quenching and low temperature tempered, carbon tool steel has a high hardness at room temperature, but the hardness was a sharp drop when the temperature was higher than 200 ℃. When the temperature reaches 500 ℃, the degree of hardness is similar to an annealing state, completely lost the ability to cut metal, which limits the application of carbon tool steel as a cutting tool. The high-speed steel makes up for the fatal defects of carbon tool steel due to its red hardness.
What is tungsten steel?
Tungsten steel (hard alloy) has a series of excellent properties such as high hardness, wear resistance, strength and toughness, heat resistance, and corrosion resistance. Especially, its high hardness and wear resistance also remain stable even under the temperature of 500 ℃, and there is still high hardness at 1000 ℃.
The main components of tungsten steel are tungsten carbide and cobalt, which account for 99% of all the components and 1% are other metals. Tungsten steel, also known as the hard alloy, is regarded as the teeth of modern industry. Tungsten steel is widely used as the material, such as a turning tool, milling cutter, drill bit, boring cutter, etc. The cutting speed of the new cemented carbide is hundreds of times that of carbon steel.
Tungsten steel is a sintered composite material containing at least one kind of metal carbide, and tungsten carbide, cobalt carbide, niobium carbide, titanium carbide, and tantalum carbide are common components of tungsten steel. The grain size of the carbide component is usually between 0.2-10 microns, and the carbide grains are bonded together with metal binders. Bonding metals are generally iron group metals, commonly used are cobalt, nickel, so there are tungsten-cobalt alloy, tungsten-nickel alloy, and tungsten-titanium cobalt alloy.
The sintering process of tungsten steel is to press the powder into the blank, then heat it into the sintering furnace to a certain temperature (sintering temperature), keep it for a certain time (holding time), and then cool it down, so as to obtain the tungsten steel material with required properties.
Conclusion
Thank you for reading our article and we hope it can help you to have a better understanding of the differences between high-speed steel and tungsten steel. If you want to learn more about tungsten and tungsten alloys, we would like to advise you to visit Stanford Advanced Materials (SAM) for more information.
As a leading supplier of tungsten products across the world, Stanford Advanced Materials (SAM) enjoys over two decades of experience in the manufacture and sale of tungsten and tungsten alloys, providing high-quality tungsten products to meet our customers' R&D and production needs. As such, we are confident that SAM will be your favorite tungsten products supplier and business partner.