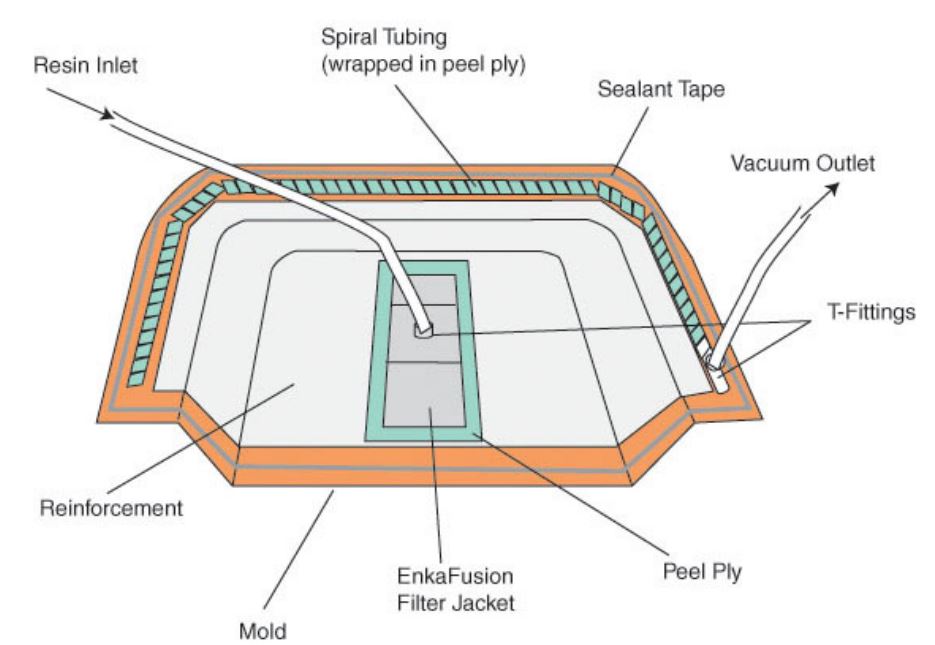
Main Factors to be considered in the Selection of Vacuum Materials
Gas permeability
The process by which a gas penetrates, diffuses, passes through and overflows a solid barrier layer from a dense side to a dense side is called infiltration.
Permeability depends on the type of gas and material. For metals, the gas permeability coefficient of some metals (such as stainless steel, copper, aluminum, molybdenum, etc.) is very small and can be ignored in most practical applications, while hydrogen has a high permeability for some metals such as iron, nickel, etc. The permeability of hydrogen to steel increases with the increase of carbon content, so it is better to choose low carbon steel as vacuum chamber material. In addition, some metals have selective permeation of gases, such as hydrogen is very easy to permeate palladium, and oxygen is easy to cast silver and so on. This property can be used for gas purification and vacuum leak detection.
The permeation of gas to glass and ceramics is usually carried out in the form of molecular state, and the permeation process is related to the diameter of gas molecules and the size of micropores inside the material. The micropore diameter of quartz glass containing pure silica is about 0.4nm, and the effective pore diameter of other glass decreases because alkali metal ions (potassium, sodium, barium, etc.) are filled in the micropore, so the gases are more permeable to quartz glass and less permeable to other glass. Since the diameter of helium molecules is the smallest among all kinds of molecules, the permeation of helium into quartz glass is the largest among gas-solid couples.
The permeation of gases to organic materials (such as rubber and plastics) is usually carried out in the molecular state. Due to the larger pores of organic materials, the permeability of gas to organic materials is much greater than that of glass and metal.
The degassing property of the material
Any solid material can dissolve and absorb some gases in the manufacturing process and in the atmosphere. When the material is placed in a vacuum, the original dynamic balance is destroyed, and the material will release air due to solubilization and desorption. The commonly used unit of exhaust gas rate is Pa * L/(s * cm2). The discharge rate is usually positive with the gas content and temperature in the material, and the unit of total gas output: Pa * L/cm2 can be used when the volume content is taken into account.
Room temperature degassing
Most organic materials are mainly composed of water vapor, which is characterized by the high abandonment rate and slow attenuation with time. Therefore, they are generally not suitable for the internal parts of vacuum containers. The normal temperature air release of glass and ceramics mainly comes from the surface, and the main air release component is water vapor, followed by CO and CO2. After baking and heating, the water vapor in the oxidation film on the surface of the glass can be basically removed, and the air release rate at room temperature can be significantly reduced.
High temperature degassing
Some structural materials, such as molybdenum electrodes, tantalum targets, boron evaporation sources, heating devices, and other equipment, are often in a high-temperature state in the process of the vacuum system. It is generally believed that the high-temperature exhalation of materials is mainly determined by the diffusion process in the body, and the amount of gas desorbed on the surface only accounts for a small part of the total exhalation. In addition to accelerating the diffusion process, high-temperature degassing of glass, ceramics, and mica is not fundamentally different from room temperature degassing. However, the diffused gas from the high-temperature body of a metal is different. Since the gas dissolved in the metal is atomic, the molecular gas emitted in a vacuum is usually formed by the surface reaction. Some metals (such as Ni and Fe) are mainly controlled by oxygen diffusion in the body. Therefore, the decarbonization of metals can reduce CO and CO2 emissions.
Glass, metal surface layer is also an important source of high-temperature gas, so the use of a variety of surface treatment processes, such as chemical cleaning, organic steam degreasing, polishing, corrosion, atmospheric baking oxidation can greatly reduce the material's gas. In addition, the rate of material degassing is not only related to the degassing time experienced, but also greatly related to the surface pretreatment method and surface condition of the material. For example, when cleaning a surface with an organic solvent to remove grease, the contamination of a single molecular layer on the surface cannot be removed and can only be removed by baking in a vacuum.

The vapor pressure and evaporation rate of the material
In vacuum technology, vapor pressure and evaporation (sublimation) rate of materials are important parameters. For example, the saturated vapor pressure of vacuum grease and vacuum regulated hot filament can be the origin of limiting vacuum degree; sublimation rate of vacuum coating materials and getter is a parameter to be considered when designing vacuum coating equipment and getter pump; the saturated vapor pressure of cryogenic liquefied gas is a parameter related to the limit pressure of cryogenic condensing pump.
Obviously, materials with high vapor pressure in the operating temperature range of the vacuum system cannot be used. In the operating temperature range, the saturated vapor pressure of all materials facing vacuum should be low enough, and the vacuum system should not fail to reach the required working vacuum degree due to its own steam pressure or the characteristics of exhaust gas. Although the vapor pressure of some materials at room temperature is low or sometimes imperceptible, the vapor pressure can eventually rise to the measured value as the temperature increases.