Understanding Catalyst Poisoning in Precious Metal Catalysts: Causes, Problems, and Solutions
1 Introduction
Precious metal catalysts play an important role in the chemical industry, the energy sector, and environmental protection. Due to their unique electronic structure and surface properties, precious metal catalysts can efficiently catalyze various chemical reactions. However, in practice, toxicants often affect these catalysts, resulting in decreased activity, altered selectivity, and even shorter lifetimes. To meet this challenge, this blog will discuss in detail the mechanisms and applications of precious metal catalysts, examine the causes and effects of catalyst poisoning, and propose measures to enhance their anti-poisoning capabilities and service life.

Fig. 1 Platinum Black Powder (Fuel Cell Grade) provided by Stanford Advanced Materials
2 Introduction to Precious Metal Catalysts
2.1 Mechanisms of Precious Metal Catalysts
In terms of electronic structure, noble metals (e.g., platinum, palladium, rhodium, iridium, etc.) have filled or nearly filled d-electron orbitals. These d-electron orbitals can effectively overlap with the orbitals of the reactant molecules, thus providing the necessary activation energy so that the reaction can be carried out at a lower energy barrier. d-electron participation enables the precious metals to form intermediates with a wide range of reactants (e.g., hydrogen, oxygen, hydrocarbons, etc.) and to facilitate the reaction process. The high electron density and uniformity of distribution of precious metal atoms give a high electron cloud density on their surfaces. This helps the noble metal catalyst to provide or accept electrons in the reaction, playing the role of a good electron donor or acceptor and promoting the reaction.
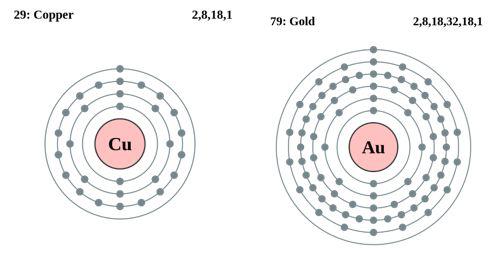
Fig. 2 Peripheral Electron Arrangements of Copper and Gold Atoms
From the perspective of surface properties, the surface of the precious metal has a strong adsorption capacity, which can effectively adsorb reactant molecules. This adsorption capacity mainly comes from the strong interaction between precious metal atoms and the high activity of surface atoms. Precious metal catalysts can interact with reactant molecules through both physical and chemical adsorption, providing active sites to promote the reaction. The surfaces of precious metal catalysts also have good reconstruction ability. During the reaction process, the surface of precious metal atoms can undergo a certain degree of reconstruction to adapt to the adsorption and reaction of different reactant molecules. This surface remodeling ability helps the catalyst maintain efficient catalytic activity under different reaction conditions.
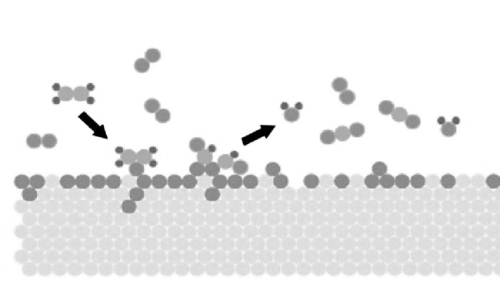
Fig. 3 Schematic Diagram of The Reaction of Gas Molecules Adsorbed on The Surface of Precious Metals
In addition, precious metals have high thermodynamic stability and can maintain their structure and catalytic activity at high temperatures and in harsh chemical environments. This enables precious metal catalysts to exhibit excellent durability and stability in a wide range of industrial reactions (e.g., high-temperature cracking, oxidation reactions, etc.).
Precious metal catalysts are capable of catalyzing many types of reactions, including hydrogenation, oxidation, disproportionation, coupling, and so on. This versatility is mainly due to their abundant surface-active sites and flexible electronic structures, which enable precious metals to adapt to different reaction mechanisms and conditions. Different kinds of precious metals can also form alloys with other metals to further regulate their electronic structures and surface properties. For example, platinum-palladium alloy catalysts exhibit catalytic performance superior to that of single metals in certain reactions. Alloying can optimize the activity, selectivity, and stability of precious metal catalysts, thus enhancing their overall performance.
2.2 Applications of Precious Metal Catalysts
Precious metal catalysts are used in gas treatment to protect the environment due to their catalytic effect on gas reactions. The ternary catalysts commonly used in automobile exhaust treatment use platinum (Pt), palladium (Pd), and rhodium (Rh) as the main components to convert carbon monoxide (CO), nitrogen oxides (NOx), and unburned hydrocarbons (HC) in the automobile exhaust into harmless carbon dioxide (CO2), nitrogen (N2), and water (H2O). Platinum and palladium are also used in diesel exhaust treatment systems to oxidize carbon particulate matter and nitrogen oxides emitted by diesel engines. Exhaust gas treatment in industrial chemical plants and refineries also uses precious metal catalysts such as platinum and palladium for treatment, which can effectively remove harmful components from the exhaust gas. Chemical sensors based on precious metal catalysts are used to detect gaseous pollutants, toxic gases, and biomolecules in the environment, e.g., hydrogen sensors, and formaldehyde sensors. Precious metal catalysts are also used for pollutant degradation, such as photocatalytic degradation of organic pollutants in water treatment, and composite catalysts of platinum and titanium oxides in photocatalytic hydrolysis of water for hydrogen production.
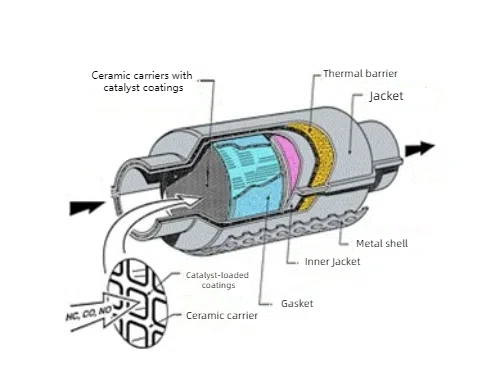
Fig. 4 Structure of The Three-Way Catalytic Converter
In the field of energy, precious metal catalysts, especially platinum catalysts, are used in the electrochemical process of water electrolysis and methanol fuel cell oxidation to enhance the efficiency of electrical energy conversion. Platinum catalysts in Proton Exchange Membrane Fuel Cells (PEMFC) promote the electrochemical reaction between hydrogen and oxygen at the electrodes to generate electricity and water. Platinum-ruthenium alloy catalysts in Direct Methanol Fuel Cells (DMFC) are used to oxidize methanol and improve fuel cell efficiency. Platinum electrodes can also be used to electrolyze water to produce hydrogen, improving the efficiency of the reaction. Precious metal catalysts are also used in biomass conversion to transform biomass feedstocks into high-value-added chemicals and fuels, such as the hydrodeoxygenation reaction in biodiesel production.
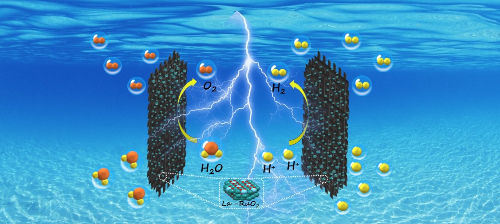
Fig. 5 Hydrogen Production from Acidic Electrolytic Water Catalyzed by La-RuO2 [5]
In chemical production, precious metal catalysts are widely used in organic-related applications. In the petroleum refining industry, both platinum and palladium catalysts can be used in the hydrodesulfurization process to remove sulfides from crude oil, which can improve fuel quality. In the oil reforming process, platinum catalysts also assist in the production of high-octane gasoline and aromatic compounds. In the organic synthesis industry, platinum and palladium catalysts are widely used to catalyze hydrogenation reactions, significantly improving the efficiency of the hydrogenation process of double and triple bonds of various organics. Palladium catalysts can also catalyze the Suzuki coupling reaction and Heck reaction, which play an important role in drug synthesis and the construction of complex organic molecules. In the field of drug synthesis, precious metal catalysts are often used for chemical transformations at key steps, such as palladium-catalyzed cross-coupling reactions, which play an irreplaceable role in the synthesis of complex drug molecules. Platinum and palladium catalysts are also commonly used in chiral catalysis, catalyzing asymmetric hydrogenation reaction processes to produce chiral drug intermediates, ensuring the optical purity and biological activity of drugs. Precious metal catalysts have important applications in the preparation of nanomaterials, such as the use of platinum and gold catalysts to prepare high-performance nanomaterials for use in electronic and optoelectronic devices.
3 Catalyst Poisoning
3.1 Definition of Catalyst Poisoning
Catalyst poisoning refers to the loss or significant reduction of catalytic activity of a catalyst during a chemical reaction due to the presence of certain substances (known as poisons or poisonous substances). These poisons strongly chemisorb or react with the active sites of the catalyst, thus preventing the catalyst from contacting and reacting normally with the reactants. Poisoning of the catalyst is an unfavorable phenomenon that leads to a reduction in the efficiency of the chemical reaction or even to its complete cessation.
3.2 Causes and Types of Catalyst Poisoning
There are three main mechanisms of catalyst poisoning.
1. Chemical Adsorption: Strong chemical adsorption of poison molecules with the active sites of the catalyst, so that these sites cannot continue to react with the reactants.
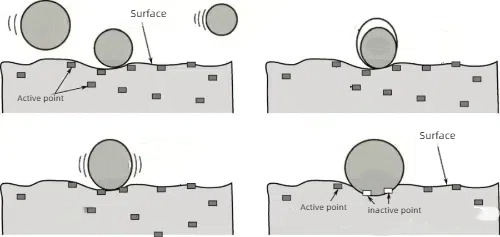
Fig. 6 Schematic Principle of Chemisorption
2. Chemical Reaction: The toxicant reacts with the active components of the catalyst to produce inactive compounds that cover the catalyst surface.
3. Physical Blockage: Certain poisons form deposits on the catalyst surface, physically blocking the pores or active sites of the catalyst.
The deactivation caused by catalyst poisoning is different due to different causes and degrees.
1. Temporary Poisoning (Reversible Poisoning): When the poison is adsorbed or chemically combined on the active center, the bond strength generated is relatively weak, and appropriate methods can be taken to remove the poison, so that the catalyst activity can be restored without affecting the nature of the catalyst, and this kind of poisoning is called reversible poisoning or temporary poisoning.
2. Permanent Poisoning (Irreversible Poisoning): The poison interacts with the active components of the catalyst to form a very strong chemical bond, and it is difficult to remove the poison in a general way to restore the activity of the catalyst, this kind of poisoning is called irreversible poisoning or permanent poisoning.
3. Selective Poisoning: After poisoning, the catalyst may lose the catalytic ability for a certain reaction, but still have catalytic activity for other reactions, this phenomenon is called selective poisoning. In a series of reactions, if the poison only causes the poisoning of the active site of the subsequent reaction, the reaction can stay in the middle stage and obtain a high yield of intermediate products.
4 Problems Caused by Catalyst Poisoning
4.1 Reduced Catalyst Activity
1. Occupation of Active Sites: Toxins strongly chemisorb or react with active sites on the catalyst surface, causing these sites to be occupied by the toxin and preventing the adsorption and reaction of reactant molecules, resulting in a significant reduction in catalyst activity. For example, sulfides (e.g., H2S) react with the surface of platinum or palladium catalysts to form platinum sulfide or palladium sulfide, thus rendering these active sites useless.
2. Surface Coverage: Toxins form a covering layer on the surface of the catalyst that physically prevents reactants from reaching the active sites of the catalyst. This covering effect also greatly reduces the effective surface area of the catalyst and decreases its activity. For example, phosphates form a covering layer on the surface of some catalysts that prevents reactants from adsorbing.
Fig. 7 Catalyst Structure Passivated After Surface Coverage
4.2 Selective Changes
1. Specific Occupancy of Active Sites
Certain toxicants selectively bind to specific active sites, resulting in modification of the activity and function of these sites. For example, certain reactions may depend on specific types of active sites (e.g., sites located on certain crystal planes or in specific atomic arrangements), and adsorption of toxicants will preferentially occupy these sites, thereby altering the overall selectivity of the catalyst.
For example, in the selective hydrogenation of ethylene, Pd catalysts exhibit high selectivity, but if the catalyst surface is poisoned by sulfur (S), sulfur atoms will preferentially adsorb to the Pd surface active sites, altering the surface properties of the catalyst and resulting in a reaction that is more inclined to produce undesired ethane rather than ethylene.
2. Alteration of The Reaction Path
The presence of a toxicant may alter the path of a catalyzed reaction, even if it does not fully occupy the active site, by changing the electronic or geometrical properties of the catalyst surface, making it more difficult for certain intermediates or transition states to form or more likely to decompose, resulting in the reaction being oriented toward a different product.
A typical example is the change in electron density on the surface of a rhodium (Rh) catalyst after poisoning of the catalyst by phosphorus (P) in the propylene hydroformylation reaction, resulting in a shift of the main product generated from n-butyraldehyde to isobutyraldehyde, a change in selectivity that is due to the different stabilizing effect of phosphorus on the reaction intermediates.
3. Surface Remodeling and Geometrical Changes
The adsorption of toxicants on the catalyst surface may cause rearrangement or remodeling of atoms or molecules on the catalyst surface, changing the geometry of the catalyst surface and thus affecting the adsorption and reaction paths of reactant molecules. Such geometrical changes may lead to a decrease or complete loss of selectivity for certain specific reactions.
In the Fischer-Tropsch synthesis reaction, iron (Fe) catalysts are used for the synthesis of long-chain hydrocarbons. Still, when the surface of the Fe catalyst is poisoned by sulfur, the sulfur atoms cause a surface remodeling that decreases the generation of long-chain hydrocarbons and increases the generation of methane and short-chain hydrocarbons. This selectivity change is due to the change in the geometric structure of the active sites on the surface.
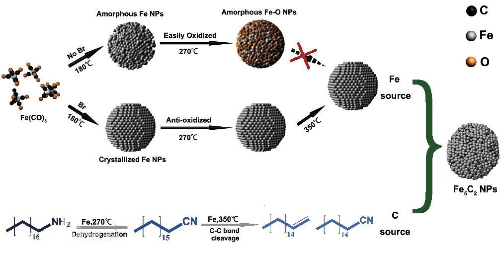
Fig. 8 Schematic Principle of The Fischer-Tropsch Process
4. Stability Changes of Intermediates
The presence of toxicants can alter the stability of reaction intermediates on the catalyst surface, resulting in easier desorption or more difficult generation of certain intermediates, thus limiting the final product distribution of the response.
In the propylene oxidation reaction, the molybdenum (Mo) catalyst is used to generate acrolein, but if the catalyst is poisoned by chlorine (Cl), the chlorine alters the stability of the reacting intermediates (e.g., propylene oxide), which results in a decrease in the selectivity of the generation of acrolein and an increase in the generation of incomplete oxidation products such as carbon dioxide.
5. Electronic Effect
Adsorption of toxicants changes the electronic environment on the catalyst surface, affecting the adsorption energy and reaction energy barriers of reactants. Especially when the toxicant is strongly electronegative or can form an electron density difference with the metal surface, this electronic effect can significantly change the reaction selectivity of the catalyst.
In the methanol partial oxidation reaction, gold (Au) catalysts are used to generate formaldehyde, but when oxygen (O2) or oxides (e.g., alumina) are present, the adsorbed oxygen atoms change the electron density on the surface of the gold catalysts, leading to the further oxidation of formaldehyde to formic acid or even carbon dioxide, which reduces the selectivity of formaldehyde.
4.3 Shortened Catalyst Life
Poisoning phenomena are often irreversible, especially when the toxicant reacts strongly with the catalyst to produce a stable compound. This irreversible deactivation means that the catalyst cannot be restored to its activity after a long period by simple treatments (e.g., regeneration), thus significantly shortening the catalyst's service life.
In addition, the action of poisons may lead to changes in the surface structure of the catalyst, or even cause agglomeration or sintering of the catalyst particles, which further reduces the stability and lifetime of the catalyst.
4.4 Increased Process Costs
As the phenomenon of toxication leads to a reduction in catalyst activity and lifetime, the process requires more frequent replacement or regeneration of the catalyst, which increases production costs. In addition, complex feedstock pre-treatments, such as desulphurization and dephosphorization, may be required before the reaction to reduce the effects of toxication, further increasing operating costs and equipment investment.
5 Measures to Address Catalyst Poisoning
5.1 Catalyst Modification
1. Alloying: Alloying is the formation of alloyed catalysts with improved properties by combining precious metals with other metals. This method is effective in improving the catalyst's resistance to toxicity. For example, palladium (Pd) is alloyed with other metals such as gold (Au) or silver (Ag) to improve its resistance to sulfur and nitrogen compounds.
Sulfides are one of the common catalyst poisons, especially in petroleum refining and chemical processes. By alloying palladium (Pd) with gold (Au) or silver (Ag), the resistance of a catalyst to sulfides can be significantly improved. For example, palladium-gold alloyed catalysts offer higher resistance to sulfide poisoning compared to pure palladium catalysts because the presence of gold alters the electronic structure of the catalyst surface and reduces sulfur adsorption, thereby slowing down the rate of poisoning.
Nitrides are also one of the main sources of catalyst poisoning, especially in ammonia synthesis and denitrification reactions. By alloying, palladium in combination with other metals such as copper Cu or platinum Pt, the tolerance of the catalyst to nitrides can be improved. Alloying can adjust the electron density and geometry of the catalyst surface, reduce the adsorption strength of nitrides, and delay the deactivation of the catalyst.
2. Surface Modification: Modification of the catalyst surface, such as adding an oxide or carbon layer to the surface of the noble metal catalyst, to prevent poisons from directly contacting the active sites. For example, oxide coating and carbon layer modification.
Adding an oxide coating, such as aluminum oxide (Al2O3) or silicon dioxide (SiO2), to the surface of a precious metal catalyst can improve the catalyst's resistance to toxicity. For example, an alumina coating on the surface of a palladium catalyst can effectively block the contact of sulfides with the active sites on the palladium surface, thus improving the sulfide resistance of the palladium catalyst. In addition, the oxide coating can provide additional acidic or basic sites, further improving the selectivity and activity of the catalyst.
Adding a carbon layer to the surface of a precious metal catalyst is also an effective method of surface modification. The carbon layer can prevent direct contact of toxicants with the active sites of the catalyst through adsorption and shielding. For example, by depositing a layer of graphene or activated carbon on the surface of a palladium catalyst, its tolerance to sulfides and nitrides can be improved while maintaining good catalytic activity. Carbon layer modification not only improves the catalyst's resistance to toxicity but also enhances its thermal stability and mechanical strength.
5.2 Pre-Treatment of Raw Materials
Feedstock pretreatment is a key step in avoiding precious metal catalyst poisoning. Through effective desulfurization, dephosphorization, and denitrogenation treatment, the effect of poison on the catalyst can be significantly reduced, the service life of the catalyst can be prolonged and its efficient catalytic performance can be maintained.
1. Desulfurization: Desulfurization refers to the removal of sulfides from the feedstock before the reaction to prevent the poisoning of the catalyst by sulfides. Sulfides are one of the common catalyst poisons, especially in petroleum refining and chemical production, where they react with the active sites on the catalyst surface, leading to catalyst deactivation. Hydrodesulfurization is a common desulfurization technology that removes hydrogen sulfide from the feedstock by reacting with sulfide at high temperature and high pressure using hydrogenated gas containing hydrogen to convert sulfide to hydrogen sulfide (H₂S). This method effectively removes organosulfur compounds such as mercaptans, thioethers, and thioesters from the feedstock, thereby reducing the toxic effect of these sulfides on the catalyst.
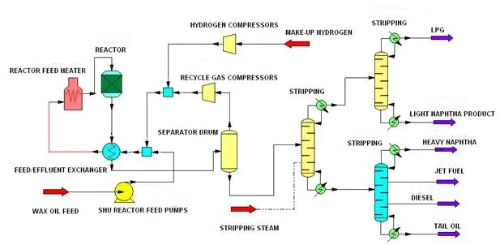
Fig. 9 Hydrodesulfurization Process
2. Dephosphorization: phosphide is also one of the main sources of catalyst poisoning, especially in certain catalytic reactions, phosphide will react with the catalyst surface, leading to the failure of the active site. Therefore, it is very necessary to use the dephosphorizing agent to dephosphorize the raw material. A dephosphorizing agent is a chemical reagent specifically designed to remove phosphide from raw material. The dephosphorizing agent reacts with the phosphides in the feedstock to form a solid precipitate that is insoluble in the feedstock and thus removes them. For example, in some industrial processes, calcium-based dephosphorizing agents can be used to react with phosphates to form calcium phosphate precipitates, thus achieving the purpose of dephosphorization.
3. Denitrogenation: Nitrogen-containing compounds are also one of the important causes of catalyst poisoning, especially in petrochemical and organic synthesis reactions, where nitrogen compounds will combine with the active sites of the catalyst, leading to catalyst deactivation. To avoid this, denitrification of the feedstock is necessary. Nitrogen-containing compounds are also one of the important causes of catalyst poisoning, especially in petrochemical and organic synthesis reactions, where nitrides will combine with the active sites of the catalyst, leading to catalyst deactivation. To avoid this situation, denitrification of raw materials is necessary.
5.3 Optimization of Reaction Conditions
1. Control the Reaction Temperature: the reaction temperature has a direct influence on the activity and stability of the catalyst. The adsorption and desorption behavior of reactants and intermediates, as well as the generation rate of poisons, will change under different temperature conditions. By optimizing the reaction temperature, the generation and adsorption of toxicants can be reduced. Performing the reaction at lower temperatures reduces the generation of certain toxic by-products. Many toxicants (e.g., sulfides, phosphides) are more readily formed at higher temperatures, and lowering the reaction temperature can inhibit the formation of these byproducts. For example, in the hydrodesulfurization (HDS) process, the production of hydrogen sulfide (H₂S) can be reduced by lowering the reaction temperature, protecting the catalyst from sulfide poisoning. Low temperatures help reduce the adsorption of poisons on the catalyst surface. High temperatures increase the kinetic energy of reactants and poisons, making it easier for them to strongly chemisorb with the active sites on the catalyst surface, which can lead to catalyst poisoning. By controlling the reaction temperature, the adsorption of poisons can be reduced and the active cycle of the catalyst can be prolonged.
2. Control Hydrogen Pressure: In hydrogenation reaction, hydrogen pressure is a key parameter, which directly affects the reaction rate and catalyst selectivity. By optimizing the hydrogen pressure, excessive hydrogenation and formation of poisons can be effectively reduced, thus protecting the precious metal catalyst from poisoning. In hydrogenation reactions, too high hydrogen pressure may lead to excessive hydrogenation of reactants and the generation of unwanted fully hydrogenated products. For example, in a partial hydrogenation reaction of alkynes, too high a hydrogen pressure can lead to over-hydrogenation of the alkynes to alkanes instead of the target product olefins. By controlling the hydrogen pressure, the degree of hydrogenation of the reactants can be precisely regulated to avoid over-hydrogenation, thereby improving reaction selectivity and protecting catalyst activity. Hydrogen pressure also affects the generation of poisons. In some reactions, excessive hydrogen pressure may promote the occurrence of side reactions and the generation of toxic by-products. For example, in the partial oxidation of methane, excessive hydrogen pressure may lead to further oxidation of formaldehyde to formic acid or carbon dioxide, thereby increasing the toxicity of poisons to the catalyst. By optimizing the hydrogen pressure, the occurrence of these side reactions can be inhibited, the formation of poisons can be reduced, and the activity of the catalyst can be protected.
5.4 Catalyst Regeneration
Catalyst regeneration is an important part of the process of avoiding the poisoning of precious metal catalysts. Catalysts will inevitably be contaminated by poisons in the course of use, which will lead to a decrease in catalytic activity. Through appropriate regeneration techniques, the poisons on the catalyst surface can be removed and its catalytic performance can be restored.
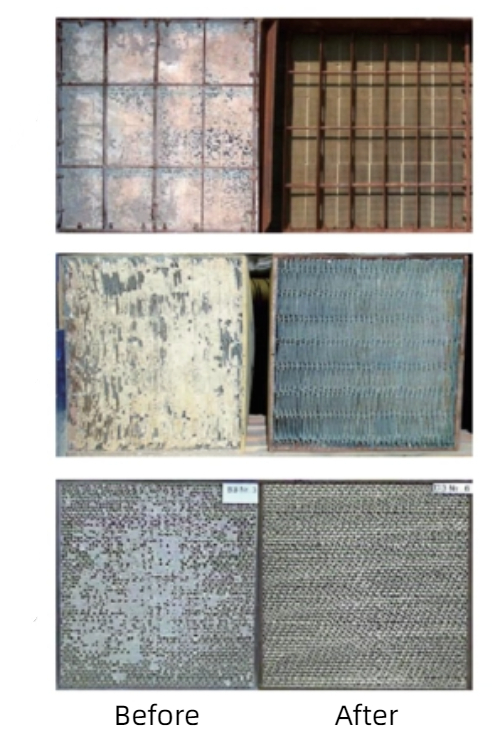
Fig. 10 Comparison of The Appearance of Various Catalysts Before and After Regeneration
1. Chemical Regeneration: Removal of poisons on the catalyst surface by chemical methods, such as oxidation or reduction treatment. This method usually includes oxidizing treatment and reducing treatment.
Oxidation treatment removes the toxicants on the catalyst surface by introducing oxygen or other oxidizing agents to oxidize and decompose the organic toxicants or other oxidizable substances on the catalyst surface. For example, for a catalyst contaminated with hydrocarbons, air or oxygen can be introduced at a high temperature to oxidize the hydrocarbons on the surface to carbon dioxide and water, thereby removing the poisons.
Reduction treatments are performed on catalysts contaminated with reducing poisons by introducing a reducing agent, such as hydrogen, to restore their activity. For example, a palladium catalyst poisoned by sulfide can be treated with reduction treatment under a hydrogen atmosphere to convert the palladium sulfide on the surface into metallic palladium and hydrogen sulfide gas, thus removing the poison and restoring the activity of the catalyst.
2. Thermal Treatment Regeneration: Remove organic poisons or coke deposits on the catalyst surface by high-temperature roasting to restore the catalyst activity. This method includes roasting and pyrolysis treatment.
High-temperature roasting is to treat the catalyst at high temperatures to remove the organic poisons or carbon deposits on the surface by thermal decomposition or combustion. For example, for a catalyst that is poisoned by coke deposits, the catalyst can be treated by roasting at a high temperature to burn off the coke on the surface, thereby removing the poisons and restoring the activity of the catalyst. The roasting temperature and time need to be optimized according to the nature of the catalyst and the type of poison to ensure the effective removal of the poison without damaging the catalyst structure.
Pyrolysis treatment removes poisons by decomposing organic poisons on the catalyst surface into volatile products at high temperatures. For example, for catalysts poisoned by organophosphates, pyrolysis treatment can be performed at high temperatures to decompose the phosphides into gaseous products, thereby removing the poisons and restoring the activity of the catalyst.
5.5 Use of Selective Toxicity Inhibitors
The addition of co-catalysts to the reaction system can also be effective in protecting precious metal catalysts. For example, the addition of small amounts of metal oxides can adsorb or convert toxicants, thereby protecting the activity of the catalyst. In palladium catalyst systems, the addition of small amounts of lanthanum (La) or cerium (Ce) oxides can significantly improve the sulfur resistance of the catalyst. These metal oxides react with the toxicants and prevent them from binding to the precious metal catalyst, thus extending the catalyst's life and maintaining its efficiency.
5.6 Advanced Catalyst Designs
1. Core-Shell Catalysts: Core-shell catalysts are a catalyst design in which the active metal core is encapsulated within a stable shell layer. A core-shell catalyst consists of a core (active metal) and a shell (protective layer). The shell is usually made of a stable material with good resistance to toxicity, such as mesoporous silica oxides, carbon materials, or alumina. The shell material enables the reactants to reach the active sites of the core by designing suitable pore sizes and channels while blocking the entry of macromolecular poisons. This structural design allows the active metal core to pass only through micropores or nanochannels when it comes into contact with the reactants, thus effectively preventing poisons from coming into direct contact and adsorbing on the active metal surface. In the case of palladium (Pd), for example, catalysts in which the palladium core is encapsulated by mesoporous silicon oxides (SiO2) can be significantly more resistant to toxication. In this structure, the palladium nucleus provides efficient catalytic activity, while the mesoporous SiO2 shell layer, through its pore size selectivity, allows small molecule reactants to enter and react with the palladium nucleus while blocking large molecule poisons, thus effectively preventing palladium nucleus poisoning.
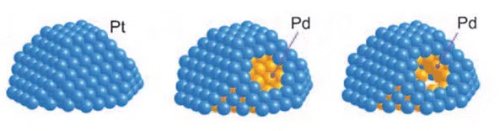
Fig. 11 The Structure of Pt-Pd Core-Shell Catalyst
2. Monoatomic Catalysts: Monoatomic catalysts are made by highly dispersing the active metal atoms on the surface of the carrier so that each active site is a single atom. This highly dispersed structure maximizes the use of metal atoms and significantly increases the activity and selectivity of the catalyst. At the same time, since each metal atom exists independently, it is difficult for poisons to aggregate on its surface, thus reducing the risk of catalyst poisoning. For example, single-atom palladium catalysts can be highly dispersed on nitrogen-doped carbon carriers. Each palladium atom is stabilized on the nitrogen-doped sites through strong interactions, and this design not only enhances the catalytic activity but also significantly improves the catalyst's resistance to poisons. Since it is difficult for poisons to aggregate around individual palladium atoms, the catalyst is significantly more resistant to toxicization.
5.7 Green Catalytic Processes
The following specific measures can be taken to reduce the toxicity problem of conventional catalysts. Firstly, use environmentally friendly solvents such as green solvents like water or supercritical CO₂ instead of toxic organic solvents to reduce the poisoning effect of poisons on the catalyst. This not only helps to improve the safety of the reaction but also reduces environmental pollution. Secondly, new catalytic systems are developed, such as research and development of emerging technologies like enzyme catalysis or photocatalysis. Enzyme catalysis realizes green chemical reactions through the high selectivity and efficiency of biological enzymes, while photocatalysis uses light energy to drive the reaction process and avoids the poisoning problem of traditional catalysts. These innovative approaches not only improve the efficiency of the reaction but also reduce the negative impact on the environment and health.
6 Conclusion
Precious metal catalysts have an irreplaceable role in the chemical industry, energy conversion, and environmental protection due to their efficient catalytic performance and wide range of applications. However, the toxicity of catalysts severely limits their long-term stable operation and application effects. Through an in-depth study of the mechanism of precious metal catalysts and the causes of the poisoning phenomenon, we can take various measures to improve the anti-poisoning ability and service life of catalysts.
First, catalyst modification, such as alloying and surface modification, can significantly improve the anti-poisoning performance of catalysts. Secondly, feedstock pretreatment and optimization of reaction conditions can effectively reduce the generation and adsorption of toxicants. In addition, catalyst regeneration and the use of selective toxicant inhibitors can help restore and maintain catalyst activity. Advanced catalyst designs, such as core-shell structured catalysts and single-atom catalysts, provide new pathways for anti-poisoning. Finally, the application of green catalytic processes not only contributes to the reduction of toxication problems but also promotes the process of sustainable development.
In conclusion, the performance and lifetime of precious metal catalysts can be significantly enhanced by the combined application of these strategies, thus meeting the demand for efficient, stable, and environmentally friendly catalysts in industrial production. Future research should continue to be devoted to the development of new anti-poisoning catalysts and green catalytic technologies to further promote the wide application of precious metal catalysts in various fields.
Related reading:
Applications of Precious Metal Catalysts: Powder vs. Pellet Insights
References:
[1] Qin T, Li N, Ma H, et al. Group VIII metals effects on lignite pyrolysis and char gasification with Ca-based catalyst[J]. Fuel,2024,372.
[2] Lysne A, Saxrud I, Snidaro L R, et al. Noble metal (Pt, Pd and Rh) promoted Ni-Co/Mg (Al)O catalysts for steam reforming of tar impurities from biomass gasification[J]. Journal of Catalysis,2024,436.
[3] Nejadmoghadam E, Achour A, Öhrman O, et al. Stabilization of fresh and aged simulated pyrolysis oil through mild hydrotreatment using noble metal catalysts[J]. Energy Conversion and Management,2024,313.
[4] Li L, Chen T, Zhang L, et al. Recent progress of Ni-based nanomaterials as promoter for enhancing the hydrogen evolution reaction performance of noble metal catalysts[J]. Journal of Alloys and Compounds,2024,998.
[5] Yun W, Rui Y, Qiang Z, et al. La-RuO2 nanocrystals with efficient electrocatalytic activity for overall water splitting in acidic media: Synergistic effect of La doping and oxygen vacancy[J]. Chemical Engineering Journal,2022,439.