PH Scale: Acids, Bases, and Common Materials
PH Scale of Common Acids
Acid | Name | 10 mM |
H2CO3 | Carbonic acid | 4.18 |
H2CrO4 | Chromic acid | 2.33 |
H2MoO4 | Molybdic acid | 2.94 |
H2S | Hydrogen sulfide | 4.47 |
H2Se | Hydrogen selenide | 2.93 |
H2SeO3 | Selenous acid | 2.47 |
H2SeO4 | Selenic acid | 1.83 |
H2SO4 | Sulfuric acid | 1.87 |
H3AsO3 | Arsenious acid | 5.58 |
H3AsO4 | Arsenic acid | 2.31 |
H3BO3 | Boric acid | 5.62 |
H3PO4 | Orthophosphoric acid | 2.26 |
H4SiO4 | Silicic acid | 5.91 |
HBr | Hydrobromic acid | 2.04 |
HCl | Hydrochloric acid | 2.04 |
HF | Hydrofluoric acid | 2.65 |
HI | Hydroiodic acid | 2.04 |
HNO2 | Nitrous acid | 2.67 |
HNO3 | Nitric acid | 2.04 |
PH Scale of Common Bases
Base | Name | 10 mM |
Ba(OH)2 | Barium hydroxide | 12.22 |
Be(OH)2 | Beryllium hydroxide | 7.90 |
Ca(OH)2 | Calcium hydroxide (lime, cao: H2O) | 12.20 |
CaCO3 | Calcium carbonate (calcite) | 9.91 |
Co(OH)2 | Cobalt(II) hydroxide | 9.15 |
Cr(OH)3 | Chromium(III)hydroxide | 7.04 |
Cu(OH)2 | Copper(II) hydroxide | 7.69 |
Fe(OH)2 | Iron(II) hydroxide (ferrous hydroxide) | 9.45 |
K2CO3 | Potassium carbonate | 11.00 |
KHCO3 | Potassium hydrogen carbonate | 8.25 |
KOH | Potassium hydroxide (caustic potash) | 11.95 |
Mg(OH)2 | Magnesium hydroxide (mgo: H2O) | 10.40 |
Na2B4O7 | Sodium borate (borax) | 9.17 |
Na2CO3 | Sodium carbonate (soda ash) | 10.97 |
Na2SiO3 | Sodium metasilicate | 11.91 |
Na3PO4 | Trisodium phosphate | 11.71 |
NaHCO3 | Sodium hydrogen carbonate | 8.22 |
NaOH | Sodium hydroxide | 11.95 |
NH4OH | Ammonium hydroxide (NH3:H2O) | 10.61 |
Ni(OH)2 | Nickel(II) hydroxide | 8.37 |
Zn(OH)2 | Zinc hydroxide | 8.88 |
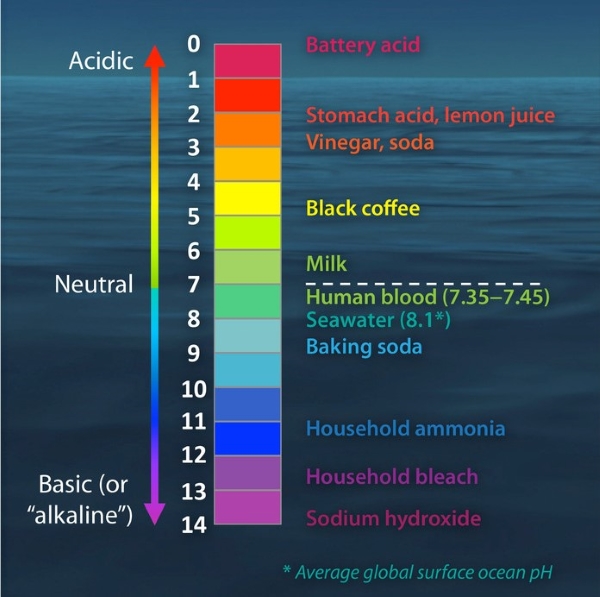
PH Scale of Common Materials
pH Value | Example |
0 | Sulfuric acid |
1 | Stomach acid |
2 | Lemon juice, vinegar |
3 | Orange juice, carbonated beverages |
4 | Tomatoes, acid rain |
5 | Black coffee, bananas |
6 | Urine, milk |
7 | Distilled water |
8 | Sea water, eggs |
9 | Baking soda |
10 | Great salt lake, milk of magnesia |
11 | Household ammonia solution |
12 | Soapy water |
13 | Household bleach, oven cleaner |
14 | Liquid drain cleaner |
PH Scale: FAQs
1. What is the pH scale?
The pH scale is a measurement system that ranges from 0 to 14 and indicates the acidity or alkalinity (basicity) of a solution. It measures the concentration of hydrogen ions in a substance. A pH of 7 is considered neutral, while values below 7 are acidic, and above 7 are basic.
2. How is pH measured?
PH is measured using a pH meter or pH paper. The meter uses a glass electrode to detect the concentration of hydrogen ions in a solution. PH paper contains indicators that change color based on the acidity or alkalinity of the substance it contacts.
3. What are acids and bases?
Acids are substances that release hydrogen ions (H⁺) when dissolved in water, increasing the concentration of these ions. Bases, on the other hand, are substances that release hydroxide ions (OH⁻) or accept hydrogen ions, reducing their concentration.
4. What are some examples of acids and bases?
Common acids include hydrochloric acid (found in the stomach), citric acid (found in citrus fruits), and vinegar (acetic acid). Bases include substances like sodium hydroxide (lye), baking soda (sodium bicarbonate), and ammonia.
5. Why is pH important?
PH is crucial in various fields like chemistry, biology, and environmental science. In the human body, different pH levels are vital for enzyme function and maintaining homeostasis. In agriculture, pH affects soil quality and plant growth. Additionally, in industries such as water treatment, pH regulation is essential for safety and efficiency.
6. How does pH impact everyday life?
PH influences the taste of foods and beverages. For instance, acidic foods like lemons taste sour, while basic substances can taste bitter or soapy. PH also affects the effectiveness of cleaning products and the health of aquatic ecosystems.
7. Can pH levels change?
Yes, pH levels can change due to various factors. Adding acids or bases, chemical reactions, biological processes, and environmental factors can alter pH levels in substances like water, soil, and even within the human body.
Reference:
[1] NOAA Pacific Marine Environmental Laboratory CO2 Program (2021). The pH scale with some common examples [Photograph]. https://www.pmel.noaa.gov/co2/file/The+pH+scale+with+some+common+examples