Lithium Tantalate vs. Lithium Niobate Wafers: A Comprehensive Comparison for Tech Enthusiasts
1 Introduction
Lithium Niobate (LiNbO3, LN) and Lithium Tantalate (LiTaO3, LT) are both multifunctional crystalline materials with excellent performance. In terms of optical properties, they have electro-optical, acousto-optical, and non-linear optical properties, and can be used as filters, etc. Lithium niobate also has a unique photorefractive effect, which allows it to be used in a new field of holographic storage. In the electrical field, in addition to electrotic-optical properties, lithium niobate, and lithium tantalate have piezoelectric and pyroelectric effects, which are widely used as piezoelectric substrates and pyroelectric detection units.
This article introduces lithium niobate and lithium tantalate materials from the crystal structure, involving their optical properties, electrical properties, application scope, and preparation process, etc., to provide you with a certain reference for the choice of application materials.

Crystal Structures of Lithium Niobate and Lithium Tantalate
LN belongs to the tripartite crystal system with the 3m point group and R3c space group and triple rotational symmetry axis, and it is classified into two kinds of lithium niobate near stoichiometric (SLN) and lithium isotactic niobate (CLN). LT also belongs to the tripartite crystal system, and belongs to the ilmenite-type structure, with the lattice being ABO3 lattice of the oxy-octahedral backbone. The crystal structure of LN and LT determines their distinctive optical properties, which have unique applications in nonlinear optics and electrophotonics.
Table 1 Crystal Structure Information
| LN | LT |
Crystal Type | Tripartite Crystal System | Tripartite Crystal System |
Lattice Constant | a=b=5.148 Å c=13.863 Å | a=5.154 Å c=13.783 Å |
Space Point Group | 3m point group R3c space group | C63vR3C point group |
3 Optical Properties of Lithium Niobate and Lithium Tantalate
The unique crystal structure of Lithium Niobate and Lithium Tantalate confers upon them distinctive optical properties. LN and LT are nonlinear optical crystals characterized by high quadratic nonlinear optical coefficients, which are pivotal in various nonlinear optical processes including frequency doubling, mixing, summing, and difference generation. They exhibit significant electro-optic coefficients, indicative of their ability to alter refractive index under applied electric fields, rendering them ideal for use in electro-optic modulators and optical switches. Furthermore, both LN and LT display birefringence, manifesting as the presence of two distinct refractive indices within the crystal, thereby exhibiting polarization-selectivity towards incident light along specific orientations. Their broad transparency window spanning the visible and infrared spectral ranges underscores their significance in applications such as optical communications and laser technology.
Lithium niobate crystals exhibit a photorefractive effect, whereby the refractive index undergoes inhomogeneous changes when subjected to intense light irradiation. Initially, this phenomenon posed challenges by disrupting phase-matching conditions and reducing frequency-doubling conversion efficiency. However, subsequent research revealed that this effect could be harnessed for holographic storage, albeit necessitating irradiation or high-temperature treatment for mitigation. Presently, the photorefractive effect serves as a fundamental tool in optical information processing, finding applications in optical storage, holographic displays, spatial modulation, all-optical time differentiation, and image processing. Nonetheless, devices utilizing these crystals may experience significant light-induced scattering, referred to as "fan" noise, at high light intensities. Additionally, the crystal's extended response time may distort information reproduction, posing challenges in meeting demands for high-quality, fast-response, and long-retention applications.
Lithium tantalate crystals have many properties similar to those of lithium niobate crystals, such as the same crystal structure, ferroelectrics at room temperature, and non-stoichiometric composition. Especially in holographic storage, LT crystals have become one of the most popular photorefractive materials for holographic storage because they have similar advantages of storage as LN crystals: massive storage, long-term stability, and repeated erasure. Although lithium tantalate and lithium niobate are of the same type, there are some differences between these two crystals, such as LT crystal is more outstanding in photorefractive resistance than LN crystal, which is more than two orders of magnitude higher than it.
Table 2 Properties of LN and LT
| LN | LT |
Melting Point | 1250℃ | 1650℃ |
Curie Temperature | 1140℃ | 610℃ |
Density | 4.64g/cm3 | 7.45g/cm3 |
Mohs Hardness | 5 | 5.5-6 |
Spectral Transmission Wavelength | 0.4-2.9um | 0.4-5.0um |
Refractive Index | no=2.286 ne=2.203 (632.8nm) | no=2.176 ne=2.180 (633nm) |
Thermal Expansion Coefficient | a11=15.4×10E-6/k a33=7.5×10E-6/k | aa=1.61×10E-6/k ac=4.1×10E-6/k |
4 Electric Properties of Lithium Niobate and Lithium Tantalate
Ferroelectricity and Piezoelectric Effect
Both Lithium Niobate (LN) and Lithium Tantalate (LT) belong to the class of ferroelectric crystals, distinguished by their unique electrical properties. Ferroelectric crystals possess ferroelectricity, wherein they can be polarized in response to an applied electric field and retain this polarization even after depolarization until subjected to an opposing electric field. This characteristic arises from their non-centrosymmetric crystal structure. Ferroelectric crystals find significant applications in electronics and optics, particularly in the development of capacitors, sensors, and memory devices.
Piezoelectric effect refers to the dielectric in a certain direction by the action of external forces and deformation, its internal polarization phenomenon, at the same time in the medium of the two opposite surfaces of the positive and negative charge. When the external force is removed, it will be restored to the uncharged state, this phenomenon is called the positive piezoelectric effect. When the direction of the force is changed, the polarity of the charge also changes. Crystals with a piezoelectric effect are called piezoelectric crystals. The cell of a piezoelectric crystal is asymmetric, but it can still exist in electrically neutral equilibrium. When pressure is applied to the surface of the crystal, the crystal structure deforms and the atoms push against each other, thus generating an electric current and completing the transformation from mechanical force to electricity; when an electric current is applied to the piezoelectric crystal, the crystal expands and contracts and the transformation from electric current to mechanical energy can be realized.
LN and LT: Superior Piezoelectric Materials
Lithium niobate crystals and lithium tantalate crystals are typical piezoelectric materials with excellent piezoelectric properties, compared with commonly used piezoelectric crystal quartz, lithium niobate crystals, and lithium tantalate crystals excellent piezoelectric effect and electromechanical coupling effect, can be prepared for high-frequency devices, so lithium niobate crystals can be used for resonators, transducers, delay lines, filters, etc., applied to mobile communications, satellite communications, digital signal processing, television sets, It is used in mobile communications, satellite communications, digital signal processing, television, broadcasting, radar, remote sensing telemetry and other civilian fields, as well as electronic countermeasures, fuzes, guidance and other military fields, of which the most widely used is the surface acoustic wave filter (SAWF), which is widely used in the field of SAW filters, piezoelectric transducers and other fields.
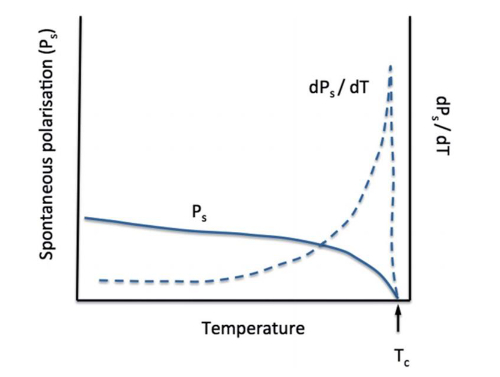
The phenomenon of change in the intensity of spontaneous polarization of a polar crystal due to a change in external temperature is called the pyroelectric effect. Crystals with this property are called pyroelectricity of crystals. A fundamental characteristic of ferroelectric crystals is that they have a pyroelectric effect, which can be produced without the addition of an additional electric field. Ferroelectric crystals, after falling below their Curie temperature, spontaneously undergo a certain degree of phase transition due to the lack of vibrational modes in the crystal's lattice, which results in asymmetry in certain directions. If the pyroelectric is heated (dT/dt>0), at which point the dipoles within the material lose their orientation due to thermal vibrations, their level of spontaneous polarization will decrease. If the material is in the open circuit state, the free charge remains on the electrode surface and generates a potential on the material. If the material is in a short-circuit state, a current is generated between the two polarized surfaces of the material. Similarly, if the pyroelectric is cooled (dT/dt < 0), the dipole will regain its orientation, which leads to an increase in the level of spontaneous polarization, whereby the free charge is attracted to the polar surfaces, thus reversing the current flow under short-circuit conditions.
5 Applications of Lithium Niobate and Lithium Tantalate
5.1 SAW Filters
Filters in SAW devices: Filters have been studied more in SAW devices. Filters have the advantages of low transmission loss, high reliability, high fabrication flexibility, analog/digital compatibility, excellent frequency selectivity, and the ability to implement a wide range of complex functions. The materials used to make filters generally require good surface flatness, high electromechanical coupling coefficients, low propagation loss, low-temperature coefficients, good repeatability, high reliability, mass production, and low cost.
The electromechanical coupling coefficients of lithium tantalate and lithium niobate are higher than those of quartz, and lithium tantalate crystals can realize a relative bandwidth of 6% to 7%, while lithium niobate can realize a relative bandwidth of 10% to 12%, but the temperature coefficients of lithium tantalate and lithium niobate are higher, and the X-cut-lithium tantalate crystals have a zero-temperature tangent so that the point of the zero-temperature coefficient can be controlled through the precise control of the tangent precision The zero-temperature coefficient point can be controlled in the room temperature range by precisely controlling the accuracy of the cut so that it can be used to fabricate high-frequency and large bandwidth filters.
The Resonator of Filters: The resonator is the basic unit of a filter, and its performance has a large impact on the performance of the filter. With the increasing demand for higher-performance filters in communication terminals, resonator-type SAW filters are widely used to solve the problems of small size, low power consumption, and low insertion loss. The basic circuit element of a resonator-type SAW filter is the resonator. The SAW excited by the fork finger transducer is reflected back and forth between the two reflection grids to form a resonance, and by adjusting the resonance frequency of the resonator and the anti-resonance frequency of the resonator, low-pass, high-pass, band-pass, and band-reject filters can be synthesized. The resonator can increase the resonant frequency and center frequency of the filter, and reduce the out-of-band rejection of the filter, resonator-type SAW filter operating frequency is generally 10MHz ~ 1GHz, with the insertion loss of 1 ~ 5dB. lithium tantalate as the center of the composite material as a resonator element Q value is higher, the generation of multiple harmonics plays a role.

5.2 Oscillators
An oscillator is a device that converts direct current energy into alternating current energy at a specific frequency, typically achieved through an oscillator circuit. Oscillators operate by converting energy between magnetic and electric fields, enabling free oscillation. They are commonly categorized into RC oscillators, LC oscillators, and crystal oscillators. Crystal oscillators utilize the piezoelectric effect, where applying voltage to the crystal wafer's poles deforms the crystal, generating a voltage across the wafer. While quartz is often employed due to its small temperature coefficient and good temperature stability, its low quartz electromechanical coupling coefficient limits its ability to achieve high frequencies and wide bandwidths in filters. In efforts to enhance oscillator performance, recent research has focused on using lithium tantalate wafers, resulting in improved device performance, miniaturization, and higher frequencies.
5.3 Pyroelectric Detectors
Pyroelectric detectors operate by exchanging heat with the surrounding environment through thermal convection, thermal conduction, and thermal radiation. The working principle involves the adsorption of electrons on the surface of pyroelectric materials, resulting in a neutral surface. When subjected to heat, the surface temperature changes, causing a variation in the material's electric dipole moment. To maintain surface neutrality, the material releases an electric charge. Pyroelectric sensors offer advantages such as high detection rates, wide operating frequencies, cost-effectiveness, simple construction, and rapid response times. The detection units of pyroelectric detectors include ceramics, single crystals, and thin films. Common ceramics include potassium tantalum niobate and lead zirconate titanate, while single crystals typically comprise lithium niobate and lithium tantalate. Thin films commonly used are lithium tantalate and lead zirconate titanate films. Lithium tantalate crystals are preferred in pyroelectric detectors due to their favorable pyroelectric coefficient, Curie point, and dielectric constant.

5.4 Q-switches
Laser Q-tuning technology is based on a special optical component: a fast intracavity optical switch generally referred to as a Q-tuning switch or Q-switch. the Q-value is an indicator of the quality of the optical resonance cavity in the laser. the higher the Q-value, the lower the required pumping threshold, and the easier the laser is to oscillate. the purpose of laser Q-tuning is to compress the pulse width and increase the peak power. The purpose of laser Q-tuning technology is to compress the pulse width and increase the peak power. At present, the commonly used Q switch includes electro-optical Q technology, acousto-optic Q technology, saturable absorption dye Q, and Cr4 + ∶ YAG saturable absorption Q. New laser Q technology is constantly being developed and applied, including active Q and passive Q combination of active and passive Q double Q technology, dual passive Q technology, Q mode-locked technology.
At present, the vast majority of nanosecond pulsed lasers are made of electro-optic Q technology, electro-optic Q technology, the core material is electro-optic Q crystal, electro-optic Q crystals commonly used electro-optic Q crystals include potassium di-deuterium phosphate crystals, lithium tantalate crystals, lithium niobate crystals, and rubidium oxide titanium phosphate crystals. Lithium tantalate crystals have stable performance, do not deliquesce, and have a high damage threshold, so they are used more often.
5.5 Holographic storage
In the 20th century, with the rapid advancement of information science and technology, the limitations of magnetic tape, disks, and CD-ROMs in meeting the escalating demand for data storage became apparent. Consequently, optical storage emerged as a promising alternative, with optical refractive holographic storage recognized as a key contender for the next generation of optical storage technology. Holographic storage offers significantly higher capacity compared to traditional one-dimensional and two-dimensional memory, with capacity scaling proportional to the third power of the reciprocal of the light wavelength.
Despite the significant advantages of photorefractive holographic three-dimensional memory, such as compact size, increased storage capacity, and faster data transfer rates, the lack of ideal photorefractive materials has been a notable challenge. While Lithium Niobate (LN) crystals with photorefractive effects have shown promise for holographic storage applications, their practical implementation is hindered by limitations including low saturation diffraction efficiency, slow response speeds, and volatility. Efforts to address these challenges have involved doping LN crystals with other elemental materials such as Fe, Mn, and In, aimed at enhancing their performance and viability for practical applications.
6 Preparation of Lithium Niobate and Lithium Tantalate
6.1 Preparation of Lithium Niobate
6.1.1 Preparation of Homocomponent Lithium Niobate
The homocomponent Lithium Niobate is often prepared by the crucible lifting method. The quality of lithium tantalate crystals is generally affected by the raw material ratio, pulling speed, seed crystal quality, crucible shape and type. The advantages of the straight-pulling method are simple equipment, easy operation and doping.
6.1.2 Preparation of Lithium Niobate with Close Stoichiometric Ratio
The two-crucible method, equipped with a continuous charging device, is the most mature and commercially viable approach for growing lithium niobate (nSLN) crystals from lithium-rich melts. However, the double-crucible method is beset by challenges including complex equipment, difficulty in controlling crystal growth, and slow growth rates due to the disparity between melt and crystal components. These factors result in low yields and expensive crystals, limiting their widespread application.
Another prevalent method is the diffusion method, wherein nSLN crystals are produced by lithium diffusion into CLN crystals within a suitable lithium-rich atmosphere, influenced by diffusion temperature and time. Optically homogeneous nSLN crystals can be obtained without inclusions or scattering particles, up to practical levels, provided the diffusion substrate exhibits high optical homogeneity. Nonetheless, most diffusion methods reported in the literature yield Z-cut nSLN wafers with small thicknesses. Thick or non-Z-cut substrates may lead to cracking or even breakage of the wafer post-diffusion treatment. In practical optical applications, larger dimensions are often required to meet through-aperture and optical path design specifications. Additionally, the diffusion method faces challenges related to wafer corrosion, recycling of lithium-rich raw materials, batch preparation, and batch consistency of crystal components, affecting overall cost efficiency.
6.2 Preparation of Lithium Tantalate
6.2.1 Preparation of Homocomponent Lithium Tantalate
Lithium Tantalate crystals of the same composition are often prepared by mixing high-purity tantalum pentoxide and high-purity lithium carbonate at a stoichiometric ratio of 0.95:1 (molar ratio) and using the crucible pulling method. The quality of lithium tantalate crystals is generally affected by the ratio of raw materials, the pulling speed, the quality of seed crystals, the shape and type of crucible, and other factors. Lithium tantalate crystals are sliced, blackened, ground, chamfered, and cleaned to obtain lithium tantalate wafers. The advantages of the straight drawing method are simple equipment, easy operation and doping. The blistering method, die-guiding method, and temperature gradient method can also accomplish the preparation of lithium tantalate crystals with the same composition, but they are less used considering the preparation cost, crystal quality, and process difficulty.
6.2.2 Preparation of Lithium Tantalate with Close Stoichiometric Ratio
It is difficult to prepare near stoichiometric lithium tantalate crystals, and the current methods of preparing near stoichiometric lithium tantalate crystals mainly include the double crucible method, the flux pulling method, the zone melting method, and the gas phase exchange equilibrium method.
Double-crucible Method: The double-crucible method is to continuously add melt to the crucible during the crystal preparation process to keep the melt composition in the crucible unchanged, so as to prepare lithium tantalate crystals with near stoichiometric ratio. The near stoichiometric lithium tantalate crystals prepared by the two-crucible method are homogeneous, but the process is complicated and costly, and the solid-liquid interfacial partitioning leads to a large number of growth fringes in the grown crystals.
Flux Pulling Method: The flux pulling method is to add flux in the crystal-melt to adjust the melting point of the crystal, and the commonly used flux is K2O. This method is less difficult, but the flux is easy to get into the crystal, and as the proportion of the flux increases, the composition of the melt changes with the growth of the crystal, and it is difficult to ensure the homogeneity of the prepared crystals.
Zone Melting Method: The zone melting method is the use of thermal energy at one end of the semiconductor bar to produce a melting zone, and then fused to a single crystal seed crystal, adjust the temperature so that the melting zone slowly moves to the other end of the bar, through the entire bar to complete the preparation of crystals. The crystals grown by this method have uniform composition distribution, energy saving, high raw material utilization, and high crystal quality.
Gas Phase Exchange Equilibrium Method: The biggest advantage of the gas phase exchange equilibrium method is that the Li content of the crystals can be controlled during the growth process, and any lithium tantalate samples with known Li content can be obtained according to the actual demand, but this method takes a long time to process the crystals and is suitable for the preparation of large-size sheet samples, and it is difficult to obtain large and uniform stoichiometric ratio of single crystals.
Table 3 Comparison of different methods of preparation of Lithium Tantalate with close stoichiometric ratio
Method | Advantages | Disadvantages |
Double-crucible method | 1. Capable of producing uniformly near-stoichiometric lithium tantalate crystals. 2. Proximity to the stoichiometric ratio of lithium tantalate crystals. | 1. Complex process, high cost. 2. Solid-liquid interface partitioning leads to a large number of growth fringes in the grown crystals. |
Flux pulling method | 1. Relatively simple process. 2. Ability to adjust the crystal's melting point. | 1. Flux easily infiltrates into the crystal. 2. As the proportion of flux increases, the composition of the melt changes with crystal growth, making it difficult to ensure crystal homogeneity. |
Zone melting method | 1. Crystals exhibit uniform composition distribution. 2. Energy-saving, high raw material utilization, and high crystal quality. | 1. Relatively complex process 2. Requires high operational skills. |
Gas phase exchange equilibrium method | 1. Control over the crystal's Li content during the growth process. 2. Ability to obtain lithium tantalate samples with known Li content according to specific demands. | 1. Lengthy processing time for crystals. 2. Suitable for preparing large-size sheet samples, challenging to obtain large and uniform stoichiometric single crystals. |
7 Conclusion
Both Lithium Niobate and Lithium Tantalate have excellent nonlinear optical and optoelectronic properties and can be used in optical devices such as filters, electro-optical devices, piezoelectric and pyroelectric components, and holographic storage. Lithium niobate may be preferred for holographic storage where higher resolution and image quality are required, while lithium tantalum is preferred in scenarios where photorefractive effects need to be minimized. In terms of preparation, the crystal growth pulling method is still the most basic preparation method and different types of LTs are prepared using different methods, each with its advantages and disadvantages, and the overall process is more complex.
As excellent optical, photovoltaic, piezoelectric, and thermoelectric materials, lithium niobate and lithium tantalate are available at Stanford Advanced Materials, and you are welcome to consult with SAM's specialists if you have more specific scenarios and questions about their use in practical applications.
Product Pages:
CY0027 Lithium Tantalate Wafers (LiTaO3 Wafers)
CY0066 Lithium Niobate Wafers (LiNbO3 Wafers)
References
[1] Xiao, X., Xu, Q., Liang, S. et al. Preparation, electrical, thermal, and mechanical properties of near-stoichiometric lithium tantalate wafers. J Mater Sci: Mater Electron 33, 20668–20677 (2022). https://doi.org/10.1007/s10854-022-08878-3
[2] KIMURA T, OMURA M, KISHIMOTO Y, et al. Comparative study of acoustic wave devices using thin piezoelectric plates in the 3~5 GHz range [J]. IEEE Transactions on Microwave Theory and Techniques, 2019, 67(3): 915-921.
[3] RUBY R, GILBERT S, LEE S K, et al. Novel temperature-composed, silicon SAW design for filter integration [J]. IEEE Microwave and Wireless Components Letters, 2021, 31(6): 674-677.