Everything You Should Know About MoO3 Nanoparticles
Introduction
Molybdenum trioxide is one of the transition metal oxides with the chemical formula MoO3(H2O)n (n=0 to 3). MoO3 is used in a wide range of applications such as photocatalysts, optics, gas sensing, batteries, electronic devices, and so on. Anhydrous MoO3 makes a distorted “MoO6” octahedra structure. Figure 1 represents its orthorhombic crystal. The green spheres are molybdenum and the red spheres are oxygen. MoO3 has 3 different crystal structures: α-orthorhombic, β-monoclinic, and h-hexagonal phases. Different MoO3 structures give them different physical and chemical properties. The h-MoO3 shows phase stability up to 436℃, but the α-MoO3 shows an irreversible phase transition under 436℃ [1].

Figure 1: MoO6 octahedra structure
How is MoO3 Nano Structure Made And Solution Combustion Synthesis Discussion
There are several synthetic ways to prepare molybdenum trioxide nanoparticles:
Hydrothermal Synthesis: molybdenum salts such as ammonium molybdate react with hydrogen peroxide under a high-temperature, high-pressure water-based solution to form MoO3 nanoparticles.
Solvothermal Synthesis: molybdenum salts react with an organic solvent such as ethanol under a high-temperature environment to form MoO3 nanoparticles.
Co-precipitation: a molybdenum salt solution reacts with a precipitation agent, such as a metal hydroxide or carbonate under a specific pH and MoO3 nanoparticles will precipitate from the solution.
Solution Combustion Synthesis: Molybdenum salts mix with a fuel-oxidizer mixture and burn under a high temperature to form MoO3 nanoparticles.
There are many other synthetic ways that are not mentioned here. For more information or interests please contain us at Stanford Advanced Materials. Even in one synthesis, different parameters will lead to different kinds of nano MoO3 structures. Let’s use Solution Combustion Synthesis as an example.
Dissolve ammonium heptamolybdate (NH4)6Mo7O24·4H2O in distilled water and mix the solution with an organic solvent (Here we use Urea, EDTA, PEG 200 and Sorbitol as different organic additives to do the experiments). Heat and stir the solution till the precipitates form. The last step is to heat the precipitates to eliminate organic additives and other impurities [2].
Ammonium heptamolybdate (AHM) is a large complex molecule that is often used as a precursor in Mo compound productions. The AHM’s solution combustion synthesis chemical equation is
MoO3 can form without using any additives, but additives play an important role in leading the crystal growth and MoO3 nuclei. Use a Scanning electron microscope (SEM) to detect the microstructures produced by using different organic additives. We get the following observations: Urea makes more spherical morphology than the other 3 additives. PEG 200 makes larger sub-micro and less spherical nanoparticles. Sorbitol and EDTA make quite different nanorods [2]. These are caused by organic additives’ chemical structure. Figure 2 below gives you these four organic additives’ chemical structures. Urea has nitrogen with a nonbinding pair of electrons. PEG200 and sorbitol have oxygen in OH group. EDTA has both nitrogens with a nonbinding pair of electrons and oxygen in OH group. In ligand formation, the nitrogen with 2 free electrons is more than oxygen. So urea is easier to attract Mo from AHM to form nuclei with a smaller size compared with PEG200 and sorbitol [2].
EDTA has 2 nitrogen with 2 free electrons and 4 oxygen in OH. It may be the most suitable additive for making MoO3 nanoparticles in the first view. But EDTA is a very large complex compound as mentioned before. The steric hinder effect prohibits EDTA’s nitrogen to attract Mo. Only oxygen group involves in ligand structure and makes microstructures of MoO3 [2].
PEG200 has only oxygen group on the two sides. It is not as attractive as urea, which means it has a low possibility to form MoO3 simultaneously on both sides. But PEG200 is a very simple structure compound with a low steric hinder effect. It is easier for PEG 200 to make ligand formation than for EDTA [2].
When one of sorbitol’s oxygen groups binds to Mo, it’s impossible for its other oxygen group to bind to other Mo due to its linear structure. In all sorbitol is not a good additive to make MoO3 nanoparticles [2]. Other conditions such as pH, reaction temperature, Mo concentration, and Mo/ Additive ratio can also affect produced MoO3 nanoparticles’ properties.
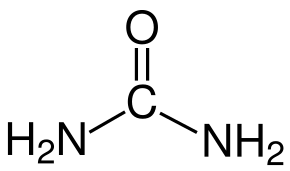
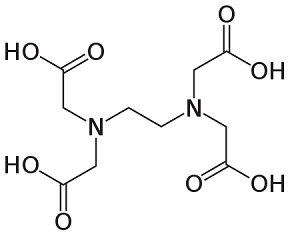
Urea EDTA
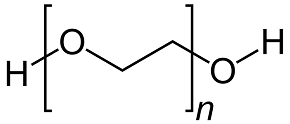
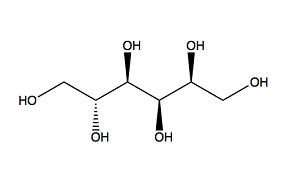
PEG200 Sorbitol
Figure 2: organic additives’ molecular structures used in the experiment
MoO3 Applications
MoO3 membrane has good electrochromic properties. Compared with other materials such as WO3 and TiO2, MoO3 has a shorter response time. Also, MoO3 changes into grey when it detects an electrical stimulus. Its absorption curve is smooth within the visible region. The absorption peak is near 550nm which is close to the human eyes’ sensitive band. How to make supreme MoO3 by MoO3 nanoparticles is one of the most popular research.
PVC is a widely used thermoplastic polymer material, but it produces dense smore when burning. The transition metal shows good smoke suppression. By combining 2 or more kinds of transition metal compounds, PVC’s dense smoke can be restricted seriously. PVC has serious fire hazards in use because of the addition of plasticizers. MoO3 also shows good flame retardation. Combining MoO3 with Cu2O, they show a synergistic effect that can reduce pure MoO3 additive cost and keep cables’ good properties.
MoO3 is a highly efficient photocatalyst. Unlike traditional wastewater dye treatment, Nano photocatalysts can convert pollutants to harmless products such as CO2 [3]. Nanoparticles give MoO3 more contacting area to make a faster degradational rate.
MoO3 is an n-type semiconductor that can be used in wide applications such as gas detection. Metal oxide gas detectors “transfer” gas into electricity which is faster and simpler than other detectors. Unlike other metal oxide gas detectors, MoO3 is a wide band-gap semiconductor material with active sites on its surface that selectively react with the gas to be measured. MoO3 has high gas-sensitive characteristics. It shows sensitivity to NH3, H2, CO, and other gases at about 450℃. Pure MoO3 membrane doesn’t work well due to its highly sensitive temperature and selectivity. Combing it with other materials to improve MoO3 gas-sensitive ability. For example, combining MoO3 with V2O5 to make membranes show high sensitivity under low temperature (about 150℃) to NO2, NH3, CO, CH4, SO2, and H2.
There are lots of applications for MoO3 nanoparticles that are not mentioned. Stanford Advanced Materials (SAM) provides different kinds of MoO3. If you want more information about MoO3, you can provide your application information to our technical staff for advice.
Reference
- Pannipa Wongkrua, Titipun Thongtem, Somchai Thongtem, "Synthesis of h- and α-MoO3 by Refluxing and Calcination Combination: Phase and Morphology Transformation, Photocatalysis, and Photosensitization", Journal of Nanomaterials, vol. 2013, Article ID 702679, 8 pages, 2013. https://doi.org/10.1155/2013/702679
- Parviz, D., Kaz→emeini, M., Rashidi, A. M., & Jafari Jozani, K. (2009). Synthesis and characterization of MOO3 nanostructures by solution combustion method employing morphology and size control. Journal of Nanoparticle Research, 12(4), 1509–1521. https://doi.org/10.1007/s11051-009-9727-6
- Thekkethil, A. J., Sreekuttan, S., & Madhavan, A. A. (2021). Application of nano molybdenum trioxide in thermal storage and photocatalysis. Journal of Physics: Conference Series, 2070(1), 012120. https://doi.org/10.1088/1742-6596/2070/1/012120