Comparative Insights into Precious Metal Catalysts: Powder vs. Pellet Forms
1 Introduction
Precious metal catalysts are essential to many reaction processes in the chemical industry due to their unique electronic structure and chemical stability. They exhibit excellent performance in terms of selectivity, synergism, and stability, and play a key role, especially in two important industrial reactors, kettle, and fixed-bed reactions. The particle size and shape of precious metal catalysts have a significant impact on reaction efficiency and product selectivity, so understanding and optimizing these parameters is essential for achieving efficient catalytic processes.
2 Characteristics of Precious Metal Catalysts
Precious metal atoms have a special activity due to the d-electrons in their outermost layers. This is reflected in the ease with which they can combine oxygen and hydrogen atoms to form covalent bonds, making the original oxidation and reduction processes easier to carry out. Therefore, precious metal monomers, oxides, and complexes can be used as catalysts. In terms of effect, precious metal catalysts are selective, synergistic, and stable.
1. Selectivity: In catalyzed reactions, there are usually multiple possible reaction pathways and product generation pathways. The selectivity of the catalyst makes a difference in the energy barriers of the different pathways and determines which major products are produced and their relative proportions under a given reaction condition. Different precious metal catalysts for the same reaction will yield different products and corresponding proportions; the same precious metal catalyst will catalyze different reactions with different results.
2. Synergistic Effect: Precious metal catalysts can be used in combination with each other so that the activity of the catalytic reaction can be greatly increased. Moreover, precious metals and other metals can form binary or multi-alloys with different morphologies and different ratios, which can not only reduce the amount of precious metals used but also improve the selectivity and service life of the catalytic reaction. Moreover, when precious metal catalysts are used in combination with different carriers, the catalytic performance obtained by different preparation methods varies greatly. It is because of the synergistic effect of precious metal catalysts that the scope of their use and research areas are also rich and colorful.
3. Stability: Precious metals are inherently chemically stable; they are not easily oxidized and will not be corroded by general acids and bases. Moreover, it has a high melting point, good thermal stability, and will not produce changes in properties under most reaction conditions. Precious metals are not susceptible to the formation of halides or sulfides under normal conditions and are therefore not easily poisoned. Precious metals may be briefly deactivated by adsorption of sulfur or CO but can be de-adsorbed and reactivated under certain conditions, and will not be permanently deactivated by the formation of stable carbonyl compounds or sulfides. On the other hand, the stability of precious metal catalysts also leads to the disadvantage that they are not easy to elute and are difficult to recover.
4. Catalytic Activity: It is the most important property that measures the catalytic efficiency of a catalyst. Compared with ordinary catalysts, the activity of precious metal catalysts is usually superior. Due to their special electronic structure and lattice morphology, noble metals can provide highly active surface active sites in catalytic reactions. These active sites are capable of adsorbing and activating reactants and lowering the energy barriers between reactants, thereby accelerating the reaction rate. The catalytic activity of precious metals combined with their high selectivity and stability makes their catalytic performance for the reaction process greatly superior to that of ordinary catalysts.
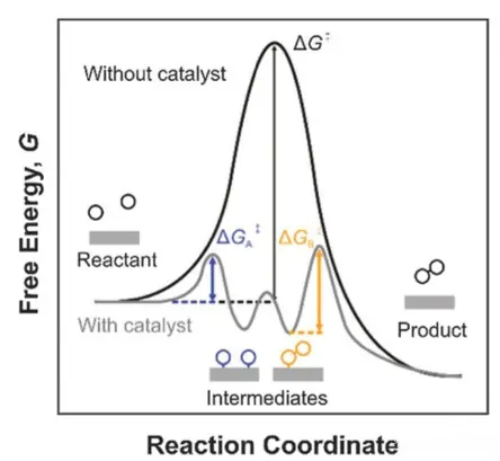
Fig. 1 Mechanism of Catalyst Action
3 Reactor Technologies: Kettle and Fixed Bed Systems
3.1 Kettle Reactors: Functionality and Catalyst Use
The kettle reactor, also known as a reaction kettle, is a kind of equipment for realizing the reaction process. It is used for realizing the single-phase reaction process of liquid phase and the multi-phase reaction process of liquid-liquid, gas-liquid, liquid-solid, gas-liquid-solid and so on. Generally, the size of the reactor is relatively large, and the amount of reaction material is large. To make full contact with the reaction material reaction, the device is often stirred (mechanical stirring, airflow stirring, etc.) device, in the high diameter is relatively large, and can be used in multi-layer stirring paddle. The kettle reactor is designed to withstand the high temperatures and pressures of the reaction process. During this process, materials may need heating or cooling. This temperature control can be achieved by installing a jacket on the reactor wall or setting up heat exchange surfaces within the device. Additionally, external circulation can be used for heat exchange, helping to control and adjust the temperature as needed.
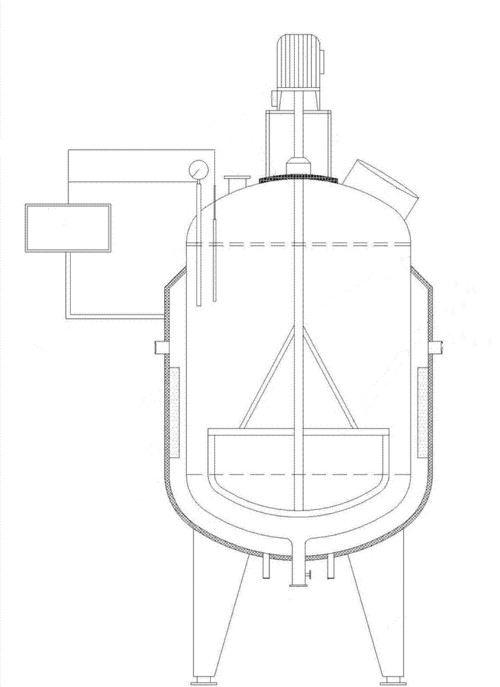
Fig. 2 Reaction Kettle Structure Schematic
The types of reactors can be divided into batch reactors and continuous reactors according to the operation mode. The batch reactor adds raw materials into the reactor once in a certain ratio and unloads the materials once after the reaction reaches a certain requirement, while the continuous operation reactor continuously adds raw materials, and continuously discharges reaction products.
Batch Reactor: The batch reactor is flexible in operation, easy to adapt to different operating conditions and product varieties, and suitable for small batches, multi-species, and long reaction times of product production. At the same time, there is no mixing of materials, which is favorable to most of the reaction. The disadvantage is that it needs auxiliary operations such as loading and unloading, and the product quality is not easy to stabilize.
Continuous Reactor: The advantages of a continuous reactor are stable product quality and ease of operation and control. The disadvantage is that there are different degrees of mixing back, which is unfavorable to most of the reactions and should be suppressed through the reactor's reasonable selection and structural design.
3.2 Fixed Bed Reactors: Performance and Catalyst Roles
Fixed bed reactor refers to the reactor filled with granular solid catalyst or solid reactants, forming a certain height of the stacked bed, gas or liquid materials through the particle gap flow through the stationary fixed bed at the same time, to achieve a non-homogeneous reaction process. It is a kind of heterogeneous catalytic reactor. This type of reactor is characterized by the solid particles filled in the equipment being fixed, different from the solid materials in the equipment movement of the moving bed and fluidized bed, also known as the filled bed reactor. Fixed-bed reactors are widely used in gas-solid phase reaction and liquid-solid phase reaction processes, such as the fixed semi-water gas generator in the ammonia industry, and the fixed-bed ion exchange column in water treatment.
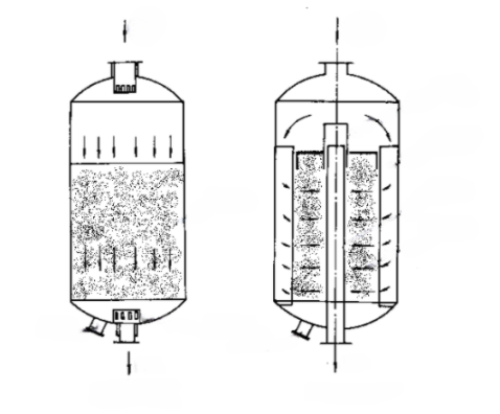
Fig. 3 Schematic Representation of Two Types of Fixed-Bed Reactors: Axial Reactor and Radial Reactor
Advantages of fixed-bed reactors include small back-mixing, effective contact between fluid and catalyst, and high selectivity when the reaction is accompanied by tandem side reactions. In addition, because the catalyst is fixed in the reactor, there is less mechanical loss of the catalyst in the mobile phase than in the kettle reaction. However, at the same time, the heat transfer of the fixed bed reactor is poor, and when the reaction exothermic heat is very large, even in the tube reactor there may be flying temperature (refers to the phenomenon that the reaction temperature is out of control and rises sharply beyond the permissible range). In the fixed-bed reactor operation process catalyst cannot be replaced, the catalyst requires frequent regeneration of the reaction is generally not applicable, often replaced by a fluidized bed reactor or moving bed reactor.
There are three basic forms of fixed-bed reactors. One is the axial adiabatic fixed bed reactor. The fluid flows through the bed from top to bottom in the axial direction, and there is no heat exchange between the bed and the outside world. The second is a radial adiabatic fixed bed reactor. Fluid flows through the bed in the radial direction, which can be centrifugal or centripetal, and there is no heat exchange between the bed and the outside world. Radial reactor and axial reactor, compared with the distance of fluid flow is shorter, the cross-sectional area of the flow channel is larger, and the pressure drop of the fluid is smaller. However, the structure of the radial reactor is more complex than the axial reactor. The above two forms are adiabatic reactors, applicable to the reaction thermal effect is not large, or the reaction system can withstand the adiabatic conditions caused by the reaction thermal effect of temperature changes in the occasion. The third is the column-tube fixed-bed reactor, which consists of several reaction tubes connected in parallel. The catalyst is arranged inside or between the tubes, and the heat carrier is heated or cooled through the tubes or inside the tubes, and the diameter of the tubes is usually between 25 and 50mm, and the number of tubes can be as many as tens of thousands. Tube fixed bed reactors are suitable for reactions with large thermal effects. In addition, there is also the above basic form of the series combination of reactors, called multi-stage fixed-bed reactors. For example: when the reaction thermal effect is large or needs to control the temperature in sections, there can be more than one adiabatic reactor in series into a multi-stage adiabatic fixed-bed reactor, heat exchanger between the reactors or supplemental materials to regulate the temperature, so as to operate close to the optimal temperature conditions.
4 Application of Precious Metal Catalysts in Reactor Technologies
4.1 Powder Form in Kettle Reactors
In chemical production, precious metal catalysts are widely used in a variety of chemical reactions due to their efficient catalytic activity and selectivity. Especially in kettle reactors, precious metal catalysts exist in powder form, providing a large specific surface area, which makes the contact between the reactants and the catalysts more adequate, thus accelerating the reaction rate. Highly dispersed precious metal powder catalysts are widely used in many organic synthesis reactions such as hydrogenation, carbonylation, and coupling reactions. These catalysts are usually prepared by mixing a precious metal precursor solution with a carrier followed by a reduction treatment. Due to their high dispersibility and large specific surface area, these powder catalysts exhibit excellent catalytic performance in kettle reactions. To further improve the efficiency of precious metal utilization, scientists have developed single-atom catalysts. These catalysts achieve extremely high catalytic efficiency and low precious metal usage by highly dispersing individual precious metal atoms on a carrier with a large specific surface area. In liquid-phase reactions, such as hydrogenation and oxidation, single-atom catalysts show comparable or even better performance than conventional nanocatalysts.
A classic example is the contact method for the manufacture of sulfuric acid. in 1831, Phillips proposed a new method for the manufacture of sulfuric acid, known as the contact method, which used platinum as a catalyst to accelerate the reaction of sulfur dioxide and oxygen to form sulfur trioxide. Although this method had been proposed early, it was not until 1875 that the contact method was industrialized through the efforts of the German chemist Maisel. This advancement marked the first large-scale industrial application of precious metal catalysts and greatly improved the productivity and purity of sulfuric acid. The realization of the contact method not only improved the efficiency and quality of sulfuric acid production but also had a profound impact on industrial technology at the time. The process could not be separated from the full contact between the reaction material and the catalyst, which was also a common idea for the realization of multiphase catalysis in later industrial processes.
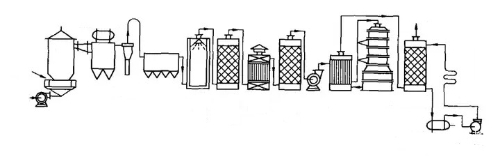
Fig. 4 The Present Contact Method Sulfuric Acid Process Flow
4.2 Pellet Form in Fixed Bed Reactors
The synthesis of vinyl acetate by gas-phase oxidation of ethylene is produced using a fixed-bed reactor unit. In this process, researchers have systematically analyzed the active layers of noble metal oxidation catalysts and explored the particle heteromorphism technique and its overall optimization for fixed-bed reactors. This suggests that the efficiency and selectivity of the reaction can be improved by changing the shape and structure of the catalyst particles in a fixed-bed reactor. The loaded Pd-Au catalyst is one of the commonly used catalysts in the synthesis of vinyl acetate by gas-phase oxidation of ethylene. To evaluate the activity of the catalysts in the study, researchers assemble fixed-bed reaction devices and study the effects of different reaction conditions on the catalytic performance. For example, the Au/Pd ratio has a significant effect on the null yield and selectivity of the catalyst. When the Au/Pd ratio was 0.86, the Pd-Au/4A catalyst showed better performance. The suitable catalyst particle size is also an important condition for ethylene gas-phase oxidative synthesis in fixed beds. The catalyst carriers suitable for ethylene gas phase synthesis of vinyl acetate generally have a particle size of about 3-7 mm, which ensures the catalyst has good mechanical strength and low-pressure drop, and at the same time facilitates the filling and reaction in the fixed bed reactor. It has been shown that the optimum specific surface area of 50-800 m²/g for catalysts of suitable particle size helps to provide more active sites, thus enhancing the catalytic effect.
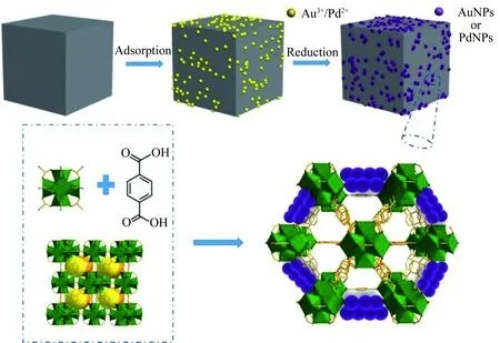
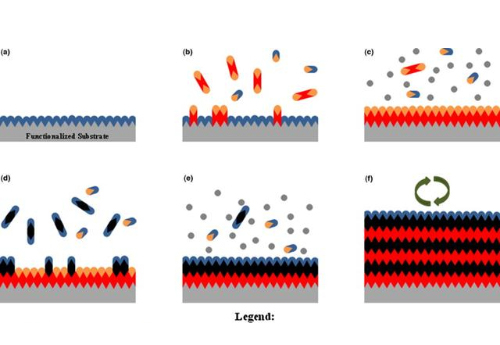
Fig. 5 Schematic Synthesis Strategy of Loaded Au-Pd Catalysts
4.3 Effect of Catalyst Particle Size on Application
In kettle reactors, uniformly dispersed catalysts are often required to ensure adequate contact between the reactants and the catalyst for efficient reactions. In terms of catalytic efficiency, catalysts in powder form provide a larger surface area, and therefore more active sites in the same volume, which can improve reaction efficiency. Moreover, kettle reactors are usually used for liquid-phase or gas-liquid-phase reactions, and catalysts in powder form can be more easily mixed with liquids or gases, thus facilitating the reaction. From the operation point of view, the kettle reactor is usually used for batch or semi-continuous operation process, in which the mixing of the catalyst in powder form and the reactants is freer and not limited by the fixed structure. Considering the reaction conditions, the powdered precious metal catalyst can be more evenly dispersed in the reaction medium, which helps to better control the reaction temperature and heat distribution and prevent localized overheating.
In a fixed bed reactor, the catalyst is usually immobilized on a carrier inside the reactor to form a catalyst bed. Granular catalysts are more suitable for this situation as they can fill the fixed bed more easily, ensure catalyst stability and mechanical strength, and provide good hydrodynamic properties. From an operational point of view, fixed bed reactors are commonly used for continuous operation processes, and granular catalysts are easy to immobilize and facilitate stability during continuous operation. Moreover, since the catalyst in a fixed bed reactor is immobile, the products of the reaction can flow directly out of the catalyst bed without additional separation steps.
Considering the reaction conditions, the fixed bed reactor is suitable for high-pressure reaction conditions because the catalyst particles can be compacted to reduce the voids in the reactor, thus improving the reaction efficiency.
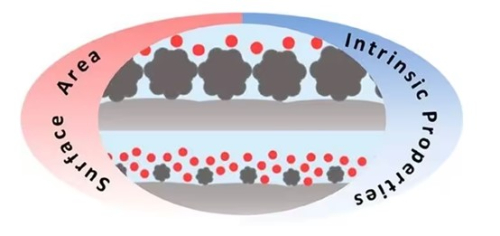
Fig. 6 Schematic Diagram of Catalyst-Particle Contact for Different Particle Sizes
5 Conclusion
Precious metal catalysts exhibit high activity, selectivity, and thermal stability in chemical reactions, making them central to chemical manufacturing processes. Their use in the kettle and fixed bed reactions not only demonstrates their broad applicability but also highlights the importance of optimizing reaction performance through catalyst design and reactor selection. Especially in key chemical processes such as the synthesis of vinyl acetate by gas phase oxidation of ethylene, the rational selection and design of precious metal catalysts is a key factor in improving reaction efficiency and product quality. In addition, the particle size and shape of precious metal catalysts directly affect the contact efficiency and catalytic activity of the reactants, requiring scientists and engineers to precisely control these parameters to achieve optimal reaction performance. Despite the many advantages of precious metal catalysts, their recovery and recycling remain a major challenge that requires further research and technological innovation to address. In conclusion, the use of precious metal catalysts in the modern chemical industry will continue to expand, presenting new opportunities and challenges. Stanford Advanced Materials (SAM) specializes in providing a wide range of high-quality, high-purity precious metal catalyst products that can be customized upon request. Browse the list of products or contact us today and one of SAM's professionals will assist you.
Related readings:
Precious Metal Catalysts: A Closer Look at the Influence of Particle Size
Common Reaction Types of Homogeneous Precious Metal Catalysts
Precious Metal Catalysts for the Petroleum Sector
Advantages of Precious Metal Catalysts
References:
[1] Gordeeva A N ,Shesterkina A A ,Vikanova V K , et al. Naphthalene and its derivatives hydrogenation for hydrogen storage: Comparative analysis of the role of noble and non-noble metal catalysts – A review[J]. International Journal of Hydrogen Energy,2024,69.
[2] Qi C X ,Lang F ,Li C , et al. Synergistic Effects of MOFs and Noble Metals in Photocatalytic Reactions: Mechanisms and Applications.[J]. ChemPlusChem,2024.
[3] Fairlie M A . Book review: The manufacture of sulfuric acid (contact process)[J]. Industrial & Engineering Chemistry,2002,18(1).
[4] Homme C A ,Othmer F D . Sulfuric Acid—Optimized Conditions in Contact Manufacture[J]. Industrial & Engineering Chemistry,2002,53(12).