Common Sulfides and Their Applications
Introduction
Sulfides have long played vital roles across diverse industries. These materials exhibit unique chemical and physical properties, lending them to applications in catalysis, energy storage, electronics, and more. This article is going to discuss some of the most commonly used sulfides and their valuable applications.
1. Lithium Sulfide (Li₂S): A Key Component in Energy Storage
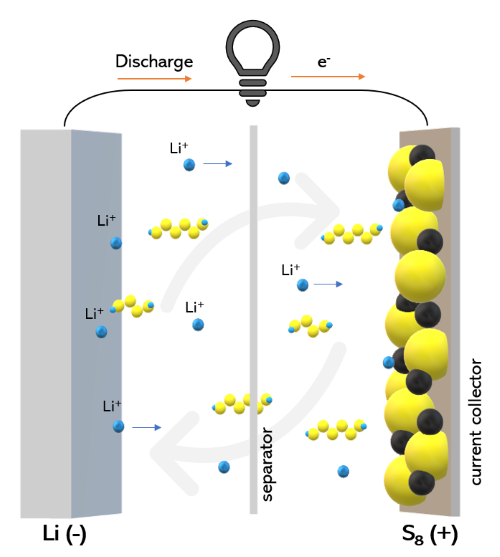
Lithium sulfide (Li₂S) is integral to lithium-sulfur (Li-S) battery technology, an emerging energy storage solution with high theoretical energy density. Li-S batteries are known for potentially storing more energy than traditional lithium-ion batteries, making them promising for electric vehicles (EVs) and grid storage. Li₂S acts as a cathode material in Li-S batteries, where its unique electrochemical reactions enable the storage and release of energy.
 [1]
[1]
Despite its advantages, lithium-sulfur batteries face challenges, including the “shuttling effect,” where intermediate lithium polysulfides dissolve in the electrolyte, reducing the battery’s lifespan. Researchers are actively developing methods to stabilize lithium sulfide within the battery, with innovations in this area moving Li-S batteries closer to commercial viability.
2. Molybdenum Disulfide (MoS₂): A Versatile Catalyst and Lubricant
Molybdenum disulfide (MoS₂) is perhaps one of the most versatile sulfides, finding applications as both a solid lubricant and a catalyst. In its natural mineral form (molybdenite), MoS₂ exhibits a layered structure, similar to graphite. This structure provides excellent lubricating properties, making MoS₂ a popular solid lubricant in heavy machinery and aerospace components, where it reduces friction and wear under high-stress conditions.

Additionally, MoS₂ serves as a catalyst in hydrodesulfurization, a key process in refining petroleum. In this application, MoS₂ removes sulfur impurities from crude oil, reducing the sulfur content in fuels and thereby decreasing emissions when they are burned. MoS₂’s catalytic abilities are due to its active edge sites, which promote sulfur-removal reactions. Research is also exploring its potential as a catalyst for hydrogen production, especially in water-splitting reactions.
3. Iron Sulfides (FeS and FeS₂): The Foundation of Metal Processing and Beyond
Iron sulfides, including iron(II) sulfide (FeS) and iron disulfide (FeS₂, commonly known as pyrite or “fool’s gold”), are used extensively in metal processing, chemical synthesis, and even in photovoltaic applications.
In metal processing, FeS is often a by-product, serving as a source of sulfur and iron for further use. Pyrite (FeS₂), meanwhile, is used in the production of sulfuric acid, an essential industrial chemical. The sulfur dioxide (SO₂) generated from roasting pyrite is converted into sulfuric acid, which is used in everything from fertilizer production to wastewater treatment.
Moreover, FeS₂’s semiconductor properties allow it to serve as a photovoltaic material. Pyrite’s natural abundance and low toxicity make it an attractive candidate for solar cell materials. However, challenges with stability and energy conversion efficiency are areas where research is ongoing.
4. Zinc Sulfide (ZnS): A Key Material for Optics and Luminescent Applications
Zinc sulfide (ZnS) is widely utilized in optics and display technologies due to its transparency in the infrared range and its ability to emit light when excited. One of ZnS’s most common uses is as a phosphor in display screens, glow-in-the-dark materials, and X-ray screens. When doped with small amounts of copper, ZnS phosphors emit light that can be tuned for various applications, creating displays that remain bright and energy-efficient.
In addition to its optical uses, ZnS also plays a role in the manufacture of infrared optics, such as lenses and windows. Because ZnS is transparent in both the visible and infrared spectra, it’s an ideal choice for these applications, especially in night vision and thermal imaging technologies.
5. Cadmium Sulfide (CdS): Applications in Photovoltaics and Electronics
Cadmium sulfide (CdS) is another significant semiconductor material, primarily used in photovoltaic cells and various electronic applications. In solar cells, CdS is often paired with cadmium telluride (CdTe) to form a highly efficient photovoltaic layer. CdS’s bandgap properties allow it to absorb sunlight effectively, making it an essential component in thin-film solar cells.
However, cadmium is a toxic element, and concerns about environmental impact have led to ongoing research into safer alternatives. Nevertheless, CdS-based thin-film solar technology remains competitive due to its high efficiency, ease of manufacturing, and scalability, driving further advancements in its design to address environmental issues.
6. Nickel Sulfide (NiS): A Catalyst in the Chemical Industry
Nickel sulfide (NiS) plays a vital role as a catalyst in chemical processes, particularly in the hydrogenation of organic compounds. NiS can catalyze reactions that add hydrogen to organic molecules, a process important in producing everything from margarine to certain pharmaceuticals. The material’s stability under harsh reaction conditions makes it an effective and durable catalyst.
Nickel sulfide also finds applications in some specialty glasses and ceramics. In glass manufacturing, NiS particles are known for their role in inducing “spontaneous breakage” in tempered glass, an area that manufacturers seek to understand better to minimize such occurrences. Although a relatively niche application, it underscores the importance of NiS’s chemical behavior and its implications for various materials.
7. Copper(I) Sulfide (Cu₂S): Conductive Films and Antibacterial Applications
Copper(I) sulfide (Cu₂S) is used in the electronics industry as a conductive material in thin films. Cu₂S films have high electrical conductivity, making them suitable for electronic devices, particularly in areas requiring transparent conductive films, such as touchscreens and other display technologies.
Cu₂S also possesses antimicrobial properties, which is particularly useful in medical devices and coatings where bacterial resistance is essential. Researchers are exploring the potential of copper sulfide nanoparticles as antimicrobial agents, particularly in healthcare settings, where they could help reduce infections and improve patient outcomes.
|
Sulfide |
Applications |
Key Properties |
|
Li-S batteries for EVs and storage |
High energy density, rechargeable |
|
|
Lubricant in machinery, catalyst for fuel refining |
Reduces friction, stable catalyst |
|
|
Iron Sulfides |
Metal processing, sulfuric acid, solar cells |
Provides sulfur, semiconductive |
|
Displays, infrared optics, glow materials |
Infrared transparent, luminescent |
|
|
Cadmium Sulfide |
Solar cells, electronics |
Light-absorbing, paired with CdTe |
|
Nickel Sulfide |
Chemical catalyst, glass ceramics |
Stable in reactions, affects tempered glass |
|
Copper(I) Sulfide |
Conductive films, antibacterial coatings |
High conductivity, antimicrobial |
Conclusion
Sulfide materials offer a remarkable array of applications. From energy storage and catalysis to electronics and optics, sulfides are integral to many cutting-edge technologies and industrial processes. Innovations in battery technology, catalysis, photovoltaics, and other fields continue to leverage the capabilities of these compounds, improving efficiency and expanding their applicability. For more information, please check Stanford Advanced Materials (SAM).
Reference:
[1] Lithium–sulfur battery. (2023, August 20). In Wikipedia. https://en.wikipedia.org/wiki/Lithium%E2%80%93sulfur_battery