Palladium on Carbon: Empowering Green Chemistry and Sustainable Synthesis
Introduction
The pursuit of sustainable and environmentally friendly chemical processes has become a driving force in modern chemistry. As researchers and industries strive to reduce their ecological footprint, catalysts that enable greener reactions have gained immense importance. Among these catalysts, palladium on carbon (Pd/C) has emerged as a powerful tool for promoting green chemistry and sustainable synthesis. In this article, we will explore how Pd/C catalysts empower green chemistry and contribute to sustainable synthesis.
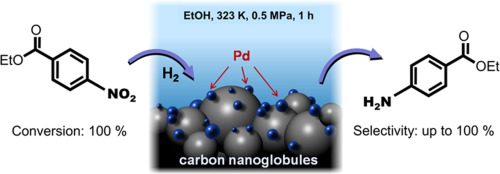
Figure 1. Palladium on Carbon
Understanding of Palladium on Carbon
Palladium on carbon catalysts are useful tools that unlock the potential of catalysis. They are indispensable in the realm of chemical synthesis for their exceptional catalytic activity, versatility in organic transformations, control over selectivity, stability, and sustainability.
Thus, Pd/C catalysts are widely used in cross-coupling, hydrogenation, carbonylation, and nitrogenation reactions. Also, such catalysts have demonstrated their ability to enable complex transformations including carbon-heteroatom bond formation reactions, decarbonylation, dehalogenation, and cyclization reactions.
Sustainability of Palladium on Carbon
Palladium on carbon catalysts get involved in green chemical reactions with the following advantages.
1. Efficient Transformations
Palladium on carbon possesses exceptional catalytic activity, enabling efficient transformations of various organic compounds. The high activity of Pd/C catalysts allows for faster reaction rates and reduced reaction times, resulting in improved process efficiency. By achieving high conversion and selectivity, Pd/C catalysts contribute to the development of sustainable synthesis routes.
2. Selectivity Control
Control over selectivity is a crucial aspect of sustainable synthesis since unwanted by-products can lead to waste generation and have negative environmental impacts. Pd/C catalysts offer excellent selectivity control, allowing chemists to direct reactions toward desired products while minimizing the formation of undesired by-products. Through the use of ligands and reaction conditions, the selectivity of Pd/C catalysts can be finely tuned, reducing waste and increasing the efficiency of the synthesis process.
3. Atom Economy
Atom economy is a fundamental principle of green chemistry that emphasizes the efficient utilization of atoms in a reaction. Pd/C catalysts contribute to atom economy by facilitating reactions that involve the selective functionalization of specific groups or positions within a molecule. This minimizes the need for excessive reagents and reduces waste formation. The high catalytic efficiency of Pd/C catalysts ensures that the maximum number of atoms in the starting materials is incorporated into the desired products, resulting in a higher atom economy.
4. Reduced Energy Consumption
Energy consumption is a significant factor in the environmental impact of chemical processes. Pd/C catalysts enable reactions to proceed under milder conditions, reducing the energy requirements for heating and maintaining the reaction. By operating at lower temperatures and atmospheric pressure, Pd/C catalysts contribute to energy savings and promote sustainable synthesis practices.
5. Catalyst Recyclability
The recyclability of catalysts is another essential aspect of green chemistry. Pd/C catalysts are known for their stability and can be easily separated from the reaction mixture and reused. The carbon support provides structural integrity to the catalyst, preventing agglomeration and maintaining its activity over multiple reaction cycles. The ability to recycle Pd/C catalysts also reduces the need for excessive catalyst loading and minimizes waste generation.
6. Minimized Environmental Footprint
The combined advantages of Pd/C catalysts, including efficient transformations, selectivity control, atom economy, reduced energy consumption, and catalyst recyclability, contribute to minimizing the environmental footprint of chemical processes. By implementing Pd/C catalysts in synthesis routes, industries can reduce waste, conserve resources, and minimize the use of hazardous reagents, which paves the way for more sustainable and environmentally friendly chemical production.
Conclusion
In a word, palladium on carbon catalysts empower green chemistry and sustainable synthesis by enabling efficient transformations, offering selectivity control, promoting atom economy, reducing energy consumption, facilitating catalyst recyclability, and minimizing the environmental footprint. As the demand for sustainable synthesis continues to grow, Pd/C catalysts play a crucial role in driving the development of greener and more efficient chemical processes. By embracing such catalysts and implementing sustainable practices, researchers and industries can contribute to a more sustainable future for the field of chemistry.
Stanford Advanced Materials (SAM) has rich experience in the manufacture and sale of Platinum on Carbon Catalysts. Precious metal catalysts are also available. Customization is welcome as well. For more information, please check our homepage.
Reference:
[1] Avelino Corma, Hermenegildo Garcia, Antonio Leyva, Catalytic activity of palladium supported on single wall carbon nanotubes compared to palladium supported on activated carbon: Study of the Heck and Suzuki couplings, aerobic alcohol oxidation and selective hydrogenation, Journal of Molecular Catalysis A: Chemical, Volume 230, Issues 1–2, 2005, Pages 97-105, https://doi.org/10.1016/j.molcata.2004.11.030.