Flammability vs Combustibility
Introduction to Flammability
Flammability is a critical property in assessing the safety and handling of materials. It determines how easily a substance can ignite and sustain combustion. Understanding flammability helps in preventing accidents and ensuring proper storage and usage of materials in various industries.
Flammability vs Combustibility
While often used interchangeably, flammability and combustibility have distinct meanings:
Flammability refers to the ability of a material to ignite quickly at lower temperatures. Flammable materials can catch fire easily and are typically more hazardous in environments where ignition sources are present.
Combustibility, on the other hand, describes materials that can burn but require higher temperatures to ignite compared to flammable substances. Combustible materials are generally considered less risky but still pose fire hazards under certain conditions.
Characteristics of Flammable Materials
Flammable materials possess specific properties that make them susceptible to catching fire:
- Low Flash Point: The temperature at which a material can vaporize to form an ignitable mixture in air is low.
- High Vapor Pressure: Indicates that the material can release vapors easily, which can ignite.
- Chemical Structure: Certain molecular structures are more prone to combustion.
Understanding these characteristics is essential for proper handling and storage to minimize fire risks.
Safety Measures for Handling Flammable Materials
Proper safety protocols are essential when dealing with flammable materials to prevent accidents:
- Storage: Keep flammable substances in approved containers away from heat sources.
- Ventilation: Ensure adequate ventilation to disperse vapors and reduce the risk of ignition.
- Protective Equipment: Use appropriate personal protective equipment (PPE) to prevent exposure.
- Fire Suppression: Equip areas with suitable fire suppression systems, such as foam or dry chemical extinguishers.
Implementing these measures helps in mitigating the dangers associated with flammable materials.
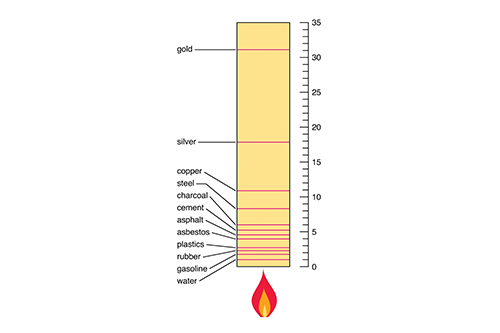
Flammable and Combustible Materials Table
Flammable Materials Table
|
Type |
Material |
Flash Point |
|
Liquids |
Gasoline |
-43°C / -45°F |
|
Ethanol (Alcohol) |
13°C / 55°F |
|
|
Acetone |
-20°C / -4°F |
|
|
Methanol |
11°C / 52°F |
|
|
Diesel Fuel |
52°C / 126°F |
|
|
Paint Thinners |
21°C / 70°F |
|
|
Benzene |
-11°C / 12°F |
|
|
Lacquer and Varnish |
15°C / 59°F |
|
|
Solids |
Charcoal Dust |
Varies by type |
|
Magnesium Powder |
Highly flammable |
|
|
Sulfur |
250°C / 482°F |
|
|
White Phosphorus |
Spontaneously ignites in air |
|
|
Gases |
Hydrogen |
-253°C / -423°F |
|
Methane |
-188°C / -306°F |
|
|
Acetylene |
-84°C / -119°F |
|
|
Propane |
-104°C / -155°F |
|
|
Butane |
-60°C / -76°F |
For more information, please check Stanford Advanced Materials (SAM).
Combustible Materials Table
|
Type |
Material |
Flash Point |
|
Liquids |
Kerosene |
38°C / 100°F |
|
Vegetable Oils |
320°C / 608°F |
|
|
Motor Oils |
180°C / 356°F |
|
|
Lubricants |
160°C - 250°C / 320°F - 482°F |
|
|
Coal Tar |
85°C / 185°F |
|
|
Solids |
Wood |
~300°C / 572°F |
|
Paper |
230°C / 446°F |
|
|
Cotton/Wool Cloth |
~250°C / 482°F |
|
|
Rubber |
~300°C / 572°F |
|
|
Plastic (PVC, PET) |
~300°C / 572°F |
|
|
Gases |
Carbon Monoxide |
N/A (combustible in certain conditions) |
|
Natural Gas |
-188°C / -306°F |
For more information, please check Stanford Advanced Materials (SAM).
Frequently Asked Questions
What is the main difference between flammability and combustibility?
Flammability refers to how easily a material can ignite at lower temperatures,
whereas combustibility indicates the ability to burn at higher temperatures.
Can a material be both flammable and combustible?
Yes, some materials can exhibit both properties depending on the conditions and
the presence of ignition sources.
Why is it important to understand flammable materials in industrial
settings?
Understanding flammable materials helps in implementing safety measures to
prevent fires and ensure the well-being of personnel and property.
What are some common examples of flammable materials?
Common flammable materials include gasoline, alcohol, acetone, and certain
types of solvents.
How can the risk of fire from flammable materials be minimized?
Risks can be minimized by proper storage, ensuring good ventilation, using
protective equipment, and having appropriate fire suppression systems in place.