Flash Point of Materials
Introduction to Flash Point
The flash point of a material is the lowest temperature at which its vapors will ignite when exposed to an open flame or spark. It is a critical property for understanding how easily a substance can catch fire, which has significant implications for safety in various industries, from chemical manufacturing to transportation, storage, and consumer product design.
The flash point is used to categorize substances into different hazard classes and to establish safety protocols for handling, storing, and disposing of potentially flammable materials. It is important to distinguish between flammable and combustible materials, as their flash points help to determine their fire risk and proper handling procedures.
Types of Flash Points
There are two main types of flash points used in the classification of materials:
Closed Cup Flash Point:
l The flash point is measured in a closed container where the material is heated, and vapors accumulate. This method simulates a worst-case scenario, where the vapors are contained and have a higher chance of igniting.
l This is the more common and conservative method used in industrial safety regulations.
Open Cup Flash Point:
l The flash point is measured in an open container. This method is generally less sensitive, as the vapor pressure is allowed to escape into the atmosphere, making it harder for a material to ignite.
l Materials with a low flash point will generally have a significantly higher flash point when measured using this method.
Flammable vs. Combustible Materials
Flash points help differentiate between flammable and combustible materials, which is crucial for safety management:
· Flammable materials: These have flash points below 100°C (212°F) and can easily ignite at relatively low temperatures. Materials like gasoline, acetone, and alcohol fall into this category. They are highly dangerous in environments where open flames, sparks, or high heat are present.
· Combustible materials: These have flash points above 100°C (212°F) and typically require higher temperatures to ignite. While they can still burn, they are considered less dangerous than flammable materials. Examples include motor oils, kerosene, and wood.
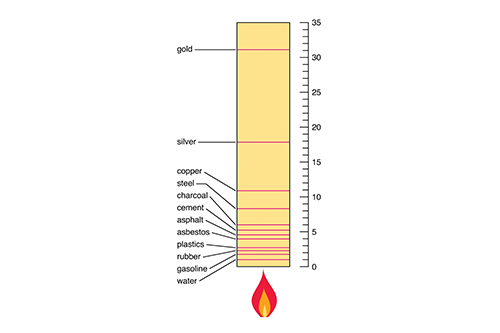
Flash Points of Common Materials
The flash point varies greatly depending on the type of material, its chemical properties, and its physical state. Below is a table that shows the flash points of some common materials:
|
Material |
Flash Point |
|
Gasoline |
-43°C / -45°F |
|
Ethanol (Alcohol) |
13°C / 55°F |
|
Acetone |
-20°C / -4°F |
|
Methanol |
11°C / 52°F |
|
Diesel Fuel |
52°C / 126°F |
|
Kerosene |
38°C / 100°F |
|
Vegetable Oils |
320°C / 608°F |
|
Motor Oils |
180°C / 356°F |
|
Paint Thinners |
21°C / 70°F |
|
Benzene |
-11°C / 12°F |
|
Wood |
~300°C / 572°F |
|
Paper |
230°C / 446°F |
|
Rubber |
~300°C / 572°F |
For more information, please check Stanford Advanced Materials (SAM).
Importance of Flash Point in Safety
1. Safety: Flash point determines safe temperature limits for handling and storage.
2. Regulations: Used in safety standards to minimize fire and explosion risks.
3. Fire Prevention: Helps prevent ignition in environments with heat or flames.
4. Firefighting: Guides firefighters in selecting proper firefighting methods.
Frequently Asked Questions
What is flash point?
Flash point is the lowest temperature at which a liquid can form an ignitable mixture with air.
Why is the flash point of oil important?
It indicates the temperature at which the oil can catch fire, essential for safety in handling and storage.
Where is flash point information found?
In safety data sheets (SDS) provided for chemicals and flammable substances.
How is flash point measured?
Using standardized testing methods like the closed cup or open cup techniques.
Can the flash point of oil change?
Yes, impurities and changes in composition can alter the flash point.