Vapor Pressure: Basics and Examples
What Is Vapor Pressure
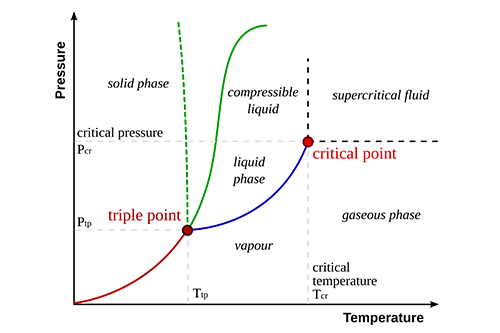
Vapor pressure is the pressure exerted by a vapor in equilibrium with its liquid or solid phase at a given temperature. It indicates how readily a substance transitions from liquid to gas.
Factors Affecting Vapor Pressure
Temperature
As temperature increases, vapor pressure rises because more molecules have the energy to escape the liquid phase.
Intermolecular Forces
Stronger intermolecular forces result in lower vapor pressure, as molecules require more energy to vaporize.
Nature of the Substance
Different substances have varying vapor pressures based on their molecular structure and bonding.
Measuring Vapor Pressure
- Manometric Method: A sealed container with a liquid is used, and the vapor pressure is measured using a manometer after the system reaches equilibrium.
- Dynamic Method: Changes in liquid volume or weight are monitored over time to calculate vapor pressure.
- Effusion Method: Measures the rate at which gas molecules escape through a small hole, which helps calculate vapor pressure.
- Clausius-Clapeyron Equation: Used to calculate vapor pressure at different temperatures.
Importance of Vapor Pressure Measurement
Measuring vapor pressure is essential in various fields, including:
- Weather forecasting: Vapor pressure is used to determine humidity and predict weather patterns.
- Industrial processes: It helps in distillation, evaporation, and other processes involving phase transitions.
- Chemical engineering: It plays a role in the design of equipment like heat exchangers, reactors, and evaporators.
- Pharmaceuticals: Vapor pressure measurements are important for the stability and storage of volatile compounds.
Examples of Vapor Pressure
Water
At 25°C, water has a vapor pressure of approximately 23.8 mmHg, indicating moderate volatility.
Acetone
Acetone exhibits a higher vapor pressure than water at the same temperature, making it more volatile.
Mercury
Mercury has a very low vapor pressure, reflecting its strong metallic bonds and low tendency to vaporize.
Vapor Pressure Table
Here’s a table of vapor pressure values for various substances at different temperatures. Vapor pressure refers to the pressure exerted by the vapor when it is in equilibrium with its liquid (or solid) phase at a given temperature.
|
Substance |
Vapor Pressure at 20°C (mmHg) |
Vapor Pressure at 100°C (mmHg) |
|
Water |
17.5 |
760 |
|
Ethanol |
44.6 |
400 |
|
Acetone |
180 |
760 |
|
Methanol |
95.3 |
1300 |
|
Benzene |
75.1 |
450 |
|
Diethyl Ether |
430 |
5800 |
|
Mercury |
0.0012 |
0.2 |
|
Hexane |
150 |
450 |
|
Toluene |
22.3 |
230 |
|
Chloroform |
160 |
500 |
For more information, please check Stanford Advanced Materials (SAM).
Frequently Asked Questions
What is vapor pressure?
Vapor pressure is the pressure exerted by a vapor when it is in equilibrium
with its liquid or solid form.
How does temperature affect vapor pressure?
Increasing temperature generally increases vapor pressure as more molecules
gain the energy to vaporize.
Why does water have lower vapor pressure than acetone?
Water has stronger hydrogen bonds, requiring more energy to vaporize compared
to acetone.
Can vapor pressure predict a substance's volatility?
Yes, higher vapor pressure indicates higher volatility and a greater tendency
to vaporize.
How is vapor pressure important in weather forecasting?
Vapor pressure contributes to humidity levels, influencing weather patterns and
precipitation.