Eutectic Point: Basics and Uses
Introduction to the Eutectic Point
The eutectic point is a fundamental concept in material science and metallurgy, representing the unique composition and temperature at which a mixture of substances solidifies simultaneously into multiple phases. This phenomenon is crucial in the development and processing of various alloys, influencing their mechanical properties and applications.
Eutectic Systems
Definition and Characteristics
A eutectic system involves a binary or multicomponent mixture where the eutectic point marks the lowest melting temperature. At this composition, the liquid transforms into two or more solid phases simultaneously upon cooling. This simultaneous solidification results in a fine microstructure, enhancing the material's properties.
Phase Diagrams
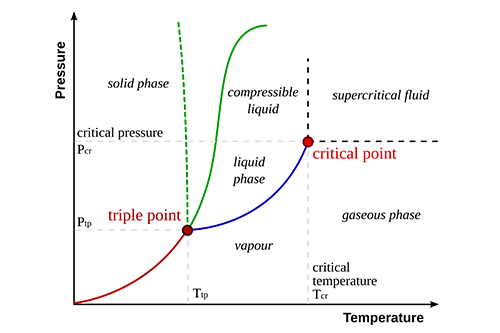
Phase diagrams are essential tools for understanding eutectic systems. They illustrate the relationship between temperature, composition, and phases present in the system. The eutectic point is typically depicted as a sharp intersection where the liquid phase transitions into two solid phases.
Applications in Material Science
Eutectic systems are widely used in soldering, where low melting points are advantageous. Additionally, they play a critical role in casting processes, ensuring uniformity and strength in the final product. Understanding eutectic systems allows engineers to tailor materials for specific applications, optimizing performance and durability.
Eutectic Alloys
Composition and Properties
Eutectic alloys are mixtures that reach the eutectic composition, providing optimal properties such as enhanced strength, ductility, and corrosion resistance. These alloys are engineered to achieve a homogeneous microstructure, which is essential for consistent performance in various applications.
Manufacturing Processes
The production of eutectic alloys involves precise control of composition and cooling rates. Techniques like alloying, casting, and solidification are employed to achieve the desired eutectic structure. Proper manufacturing ensures that the eutectic point is maintained, resulting in superior material properties.
Industrial Applications
Eutectic alloys are prevalent in industries such as automotive, aerospace, and electronics. They are used in engine components, structural materials, and electronic soldering, where their unique properties contribute to the efficiency and reliability of products. The ability to customize eutectic alloys makes them invaluable in advancing technology and engineering solutions.
Comparison of Eutectic and Non-Eutectic Alloys
|
Feature |
Eutectic Alloys |
Non-Eutectic Alloys |
|
Melting Behavior |
Single melting point |
Multiple melting points |
|
Microstructure |
Fine, uniform microstructure |
Coarse or mixed microstructures |
|
Mechanical Properties |
Enhanced strength and ductility |
Variable properties based on composition |
|
Applications |
Soldering, casting, electronics |
Structural components, tooling |
|
Manufacturing Process |
Precise composition control |
Flexible composition ranges |
Frequently Asked Questions
What is the eutectic point?
The eutectic point is the specific temperature and composition at which a
mixture of substances solidifies into multiple phases simultaneously.
Why are eutectic alloys important in industry?
Eutectic alloys offer enhanced mechanical properties and uniform
microstructures, making them ideal for applications like soldering, casting,
and electronics.
How is the eutectic point determined?
The eutectic point is identified using phase diagrams, which map the
relationship between temperature, composition, and phases in a system.
Can eutectic systems have more than two components?
Yes, eutectic systems can involve multiple components, but binary systems are
the most commonly studied and applied.
What are some common examples of eutectic alloys?
Common eutectic alloys include solder composed of tin and lead, and the
aluminum-silicon alloy used in automotive engine components.