Critical Temperature and Pressure for Common Materials
What Is Critical Temperature
Definition and Significance
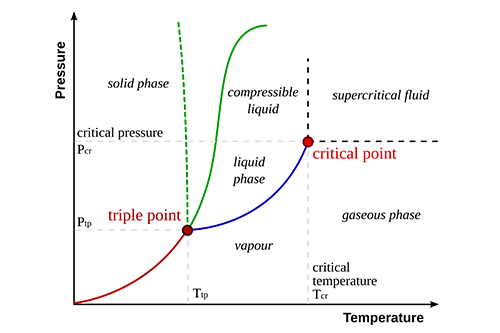
Critical temperature is a fundamental concept in chemistry and thermodynamics. It refers to the highest temperature at which a substance can exist as a liquid, regardless of the pressure applied. Beyond this temperature, the kinetic energy of the molecules overcomes the intermolecular forces, preventing the substance from condensing into a liquid phase. Understanding critical temperature is essential for various industrial applications, including the design of equipment for chemical processing and the study of phase transitions in materials science.
Critical Temperature vs. Boiling Point
While both critical temperature and boiling point involve phase changes, they are distinct concepts. The boiling point of a substance is the temperature at which its vapor pressure equals the external pressure, allowing it to transition from liquid to gas. In contrast, the critical temperature is the threshold beyond which the liquid phase cannot exist, no matter how much the pressure is increased. This means that above the critical temperature, a substance cannot be liquefied by pressure alone and exists only as a supercritical fluid.
Factors Affecting Critical Temperature
Molecular Size and Interactions
The critical temperature of a substance is influenced by the size of its molecules and the strength of intermolecular interactions. Larger molecules with stronger intermolecular forces, such as hydrogen bonding or dipole-dipole interactions, typically have higher critical temperatures. These stronger forces require more energy (higher temperature) to overcome, thus increasing the critical temperature.
Pressure Influence
Pressure plays a significant role in determining the critical temperature. At higher pressures, molecules are forced closer together, enhancing intermolecular interactions and increasing the critical temperature. However, the critical temperature itself is defined independently of pressure, representing the maximum temperature at which a substance can exist as a liquid under any pressure.
Critical Temperatures and Pressures of Common Substances
The following table provides the critical temperatures and pressures of various common substances, illustrating the diversity in their thermal and pressure-related properties.
|
Substance |
Critical Temperature (°C) |
Critical Pressure (atm) |
|
Water |
374 |
218 |
|
Carbon Dioxide |
31 |
73 |
|
Methane |
-82 |
46 |
|
-147 |
34 |
|
|
Oxygen |
-118 |
49 |
|
Ethanol |
240 |
63 |
|
Ammonia |
132 |
112 |
|
Sulfur Dioxide |
157 |
78 |
|
Benzene |
289 |
48 |
|
Acetone |
235 |
47 |
Frequently Asked Questions
What happens to a substance above its critical temperature?
Above its critical temperature, a substance cannot be liquefied by pressure alone and exists as a supercritical fluid, exhibiting properties of both liquids and gases.
How is critical temperature measured?
Critical temperature is determined experimentally by gradually increasing the temperature of a substance under controlled pressure until the liquid and gas phases become indistinguishable.
Why is critical temperature important in industrial applications?
Critical temperature is crucial for designing equipment and processes that involve phase transitions, such as supercritical fluid extraction and the operation of high-pressure reactors.
Can the critical temperature be altered by changing molecular structure?
Yes, modifying the molecular structure, such as altering functional groups or chain length, can influence the strength of intermolecular forces and thereby change the critical temperature.
Is there a relationship between critical temperature and critical density?
Yes, critical density is the density of a substance at its critical temperature and pressure, and it provides insight into the fluid's behavior near the critical point.