Coefficient of Thermal Expansion
Coefficient of Thermal Expansion
The coefficient of thermal expansion (CTE) describes the degree to which a material's size changes with temperature. It is typically expressed in units of per degree Celsius (°C⁻¹) or per Kelvin (K⁻¹). While the exact mathematical representation of CTE involves formulas, the concept revolves around the linear, area, or volumetric changes a material undergoes as temperature varies.
Factors Affecting Thermal Expansion
Several factors influence the coefficient of thermal expansion in materials:
Material Composition
Different materials have inherently different CTEs. Metals, ceramics, polymers, and composites each respond uniquely to temperature changes based on their atomic and molecular structures.
Temperature Range
The CTE can vary with temperature. Some materials exhibit linear expansion over certain temperature ranges, while others may have non-linear behaviors at higher or lower temperatures.
Structural Anisotropy
Anisotropic materials, which have direction-dependent properties, can expand differently along various axes. This is particularly important in materials like wood or certain crystals.
External Stresses
Pre-existing stresses within a material can affect how it expands or contracts when the temperature changes. Residual stresses from manufacturing processes can alter the effective CTE.
Environmental Factors
Exposure to different environments, such as humidity or chemical exposure, can influence the thermal expansion properties of materials over time.
Thermal Expansion of Common Materials
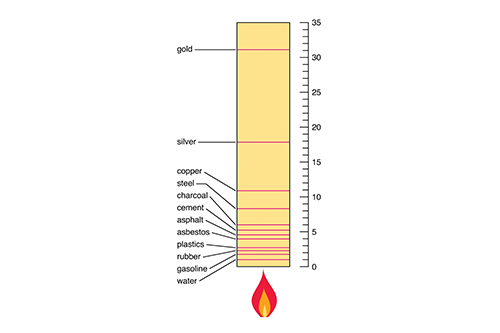
The table below provides examples of various materials and their respective coefficients of thermal expansion:
|
Material |
Coefficient of Thermal Expansion (°C⁻¹) |
|
23 × 10⁻⁶ |
|
|
Steel |
12 × 10⁻⁶ |
|
Glass |
9 × 10⁻⁶ |
|
Concrete |
10 × 10⁻⁶ |
|
Copper |
16.5 × 10⁻⁶ |
|
Brass |
19 × 10⁻⁶ |
|
8.6 × 10⁻⁶ |
|
|
Polyethylene |
100 × 10⁻⁶ |
|
Carbon Fiber |
0.5 × 10⁻⁶ |
|
Invar (Alloy) |
1.2 × 10⁻⁶ |
Thermal Expansion of Common Metals
|
Metal |
CTE (10⁻⁶ /°C) |
|
Aluminum |
23.1 |
|
Brass |
19–21 |
|
Bronze (Phosphor) |
17.6 |
|
Copper |
16.5 |
|
Gold |
14.2 |
|
Iron |
11.8 |
|
Lead |
28.9 |
|
Magnesium |
25.2 |
|
Nickel |
13.3 |
|
8.8 |
|
|
Silver |
19.5 |
|
Stainless Steel (304) |
16.0 |
|
Stainless Steel (316) |
15.9 |
|
Steel (Carbon) |
11.7–13.0 |
|
Tin |
22.0 |
|
Titanium |
8.6–9.4 |
|
4.5 |
|
|
Zinc |
30.2 |
|
Zirconium |
5.7 |
Frequently Asked Questions
What is the significance of the coefficient of thermal expansion in engineering?
The coefficient of thermal expansion is crucial in engineering for designing structures and components that can withstand temperature changes without experiencing excessive stress or deformation. It ensures the integrity and longevity of materials used in various applications.
How does the coefficient of thermal expansion affect everyday objects?
Everyday objects like bridges, railways, and buildings expand and contract with temperature changes. Understanding their CTE helps in designing expansion joints and other features that accommodate these movements, preventing structural damage.
Can the coefficient of thermal expansion be negative?
Yes, some materials exhibit negative thermal expansion, meaning they contract when heated. These materials are relatively rare and are of interest for specialized applications where controlled contraction is desirable.
How is the coefficient of thermal expansion measured?
CTE is typically measured using techniques like dilatometry, where the change in length or volume of a material is monitored as it is heated or cooled under controlled conditions.
Does the coefficient of thermal expansion vary with material purity?
Yes, impurities and alloying elements can significantly affect a material's CTE. Pure materials often have different expansion characteristics compared to their alloyed counterparts.