Segregation in Alloy Casting: Types, Causes, and Mitigation
Introduction
Segregation in alloy casting is a critical phenomenon that affects the quality and performance of metals and alloys. It refers to the non-uniform distribution of alloying elements during the solidification process. This non-uniformity can lead to variations in mechanical, chemical, and physical properties, often resulting in inferior performance.
Understanding the types, causes, and mitigation strategies of segregation is essential for producing high-quality alloys.

Types of Segregation
- Microsegregation:
Microsegregation occurs on a microscopic scale within individual grains or between dendritic arms. During solidification, the solute elements tend to concentrate in the last regions to solidify, often at the grain boundaries or interdendritic regions. This type of segregation can lead to local variations in composition, which can affect the microstructural and mechanical properties of the alloy.
- Macrosegregation:
Macrosegregation happens on a macroscopic scale, where the composition varies across the entire casting or ingot. It is often visible to the naked eye and can result in large-scale variations in the alloy’s properties. Macrosegregation typically occurs due to the movement of the liquid phase during solidification, leading to an uneven distribution of solute elements.
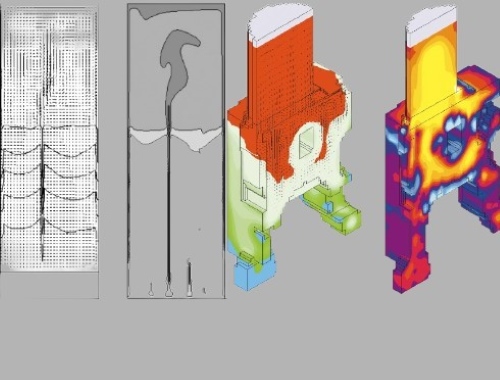 [1]
[1]
Causes of Segregation
1. Partition Coefficient (k):
The partition coefficient, defined as the ratio of the solute concentration in the solid phase to that in the liquid phase, plays a significant role in segregation. If the partition coefficient is less than one (k < 1), the solute tends to concentrate in the liquid phase during solidification, leading to segregation. For example, in an aluminum-copper alloy, copper tends to concentrate in the liquid phase, causing segregation as the alloy solidifies.
2. Solidification Rate:
The rate at which an alloy solidifies influences the extent of segregation. Rapid cooling traps solute elements in the liquid phase, creating a concentration gradient. Conversely, slow cooling allows more time for solute redistribution, potentially reducing microsegregation but increasing the risk of macrosegregation due to longer diffusion paths.
3. Density Differences:
Differences in density between solute and solvent elements can lead to gravity-induced segregation. Heavier elements may settle at the bottom of the melt, while lighter elements rise to the top. This can create significant compositional gradients within the solidified alloy.
4. Thermal Gradients:
Non-uniform temperature distribution during solidification can cause directional solidification, where the composition varies along the solidification front. Thermal gradients drive fluid flow within the melt, which can further exacerbate segregation.
5. Fluid Flow and Convection:
Natural or forced convection within the molten alloy can transport solutes, leading to segregation patterns. Fluid flow driven by thermal gradients, mechanical stirring, or electromagnetic forces can cause uneven distribution of alloying elements.
Mitigation Strategies
1. Control of Cooling Rate:
Adjusting the cooling rate is a primary method to mitigate segregation. By optimizing the cooling rate, it is possible to balance between too rapid and too slow solidification. Controlled cooling profiles can help maintain uniform solute distribution. For instance, in directional solidification techniques, a controlled thermal gradient is applied to manage solute distribution effectively.
2. Stirring or Electromagnetic Processing:
Mechanical stirring or electromagnetic stirring can enhance solute mixing within the melt. Mechanical stirring involves physically agitating the molten alloy to promote a homogeneous distribution of solutes. Electromagnetic stirring uses electromagnetic fields to induce fluid flow within the melt, enhancing solute distribution and reducing segregation.
3. Grain Refinement:
Adding nucleating agents or grain refiners can promote the formation of fine, equiaxed grains, which can reduce the extent of segregation. For example, in aluminum alloys, titanium or boron can be added to refine the grain structure, leading to a more uniform distribution of solute elements.
4. Directional Solidification Techniques:
Directional solidification techniques, such as zone refining, can help manage solute distribution. In zone refining, a molten zone is moved through the solid alloy, allowing for the redistribution of solutes and reducing segregation. Gradient control during solidification can also be used to achieve more uniform composition.
5. Homogenization Heat Treatment:
Post-solidification heat treatment, known as homogenization, can promote the diffusion of solutes, evening out compositional differences caused by segregation. This process involves heating the solidified alloy to a temperature where diffusion is significant but below the melting point, allowing for the redistribution of solutes.
6. Use of Alloys with Similar Melting Points:
Selecting alloying elements with similar melting points can reduce the tendency for segregation. Alloys with closely matched melting points tend to solidify more uniformly, minimizing compositional gradients.
Related Cases and Reports on Segregation in Alloy Casting
Here are a few notable cases and reports that highlight the significance of addressing segregation in various industrial and research settings:
Case 1: Aerospace Industry – Titanium Alloys
"Control of Macrosegregation in Large Titanium Alloy Ingots" written by J. D. Cotton, M. G. Burke details how optimized vacuum arc remelting (VAR) processes and electromagnetic stirring techniques were implemented to reduce macrosegregation in titanium alloy ingots. The study demonstrated that by controlling the solidification parameters and using advanced stirring methods, the uniformity of the alloy composition could be significantly improved, leading to better mechanical properties in the final aerospace components.
Case 2: Automotive Industry – Aluminum Alloys
"Mitigation of Microsegregation in High-Strength Aluminum Alloys for Automotive Applications" focused on the issue of microsegregation in aluminum-copper alloys. The researchers investigated the effect of different cooling rates and homogenization treatments on the microsegregation patterns. They found that a combination of rapid cooling and subsequent homogenization heat treatment effectively reduced microsegregation, resulting in more uniform mechanical properties. These findings have been applied in the production of lightweight automotive components with enhanced performance and durability.
Case 3: Additive Manufacturing – Metal 3D Printing
"Microsegregation Control in Additively Manufactured Alloys" by A. D. Rollett, T. DebRoy explored the microsegregation phenomena in various additively manufactured alloys, including titanium and aluminum alloys. The researchers investigated the effects of different AM process parameters, such as laser power and scanning speed, on microsegregation. They found that optimizing these parameters, along with post-processing heat treatments, could significantly reduce microsegregation. The findings have been instrumental in improving the quality and performance of additively manufactured metal components, making them more viable for critical applications in aerospace, medical, and automotive industries.
Conclusion
Segregation in alloy casting significantly impacts the performance and reliability of metals and alloys. By understanding the types and causes of segregation, metallurgists can implement effective mitigation strategies to produce high-quality materials.
Control of cooling rates, mechanical and electromagnetic stirring, grain refinement, directional solidification techniques, homogenization heat treatment, and careful alloy selection are all essential tools in managing and reducing segregation. These strategies must be tailored to specific alloy systems and applications to achieve optimal results, ensuring the production of alloys with uniform properties and enhanced performance. For more information, please check Stanford Advanced Materials (SAM).
Reference:
[1] K. J. B. R. W. C. .. P. V. (2001). Encyclopedia of Materials: Science and Technology. https://www.sciencedirect.com/referencework/9780080431529/encyclopedia-of-materials-science-and-technology