How to Prevent Catalyst Deactivation?
Introduction
Catalysts are essential in many industrial processes, as they enable chemical reactions to occur more efficiently and at lower temperatures or pressures. Nevertheless, catalysts can be deactivated over time, which can lead to reduced efficiency and increased costs. In this article, we will discuss how to prevent catalyst deactivation. Hope that you can have a better comprehension of the maintenance of different catalysts.
How to Prevent Catalyst Deactivation?
--Poisoning
The primary cause of catalyst deactivation is poisoning. It refers to the reversible or irreversible chemical deactivation of a catalyst and leads to the loss of catalytic activity, stability, and selectivity, which causes severe problems and economic losses in industrial catalytic processes. Figure 1. shows the sulfur poisoning by H2S of nickel catalysts with & without oxygen addition.
You can choose pretreatment or removal to prevent catalyst poisoning.
- If it is reversible, the catalyst could be reused.
- If not, the catalyst must be thrown, and a large amount of energy and expense is wasted. However, you can apply some pretreatment to the catalysts. For instance, using ZnO and other guards could effectively mitigate sulfur poisoning.
- Remove the deactivated catalysts if the total removal of poisons is rather hard.
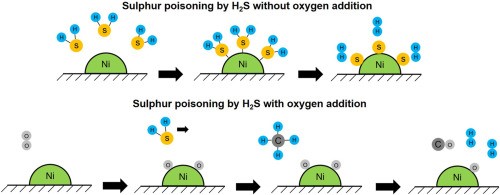
[1]
Figure 1. Sulfur Poisoning
--Sintering
Sintering is another common cause of catalyst deactivation. It is a thermal degeneration that comes with reduced catalytic surface area and support area. What’s worse, the catalytic phases would shift into non-catalytic phases, thus hindering the intended chemical reactions.
Please take caution about materials and environments to prevent sintering.
- Alkali metals speed up sintering, whereas oxides of Ba, Ca, or Sr decrease the sintering rate. Porous substances typically exhibit lower sintering rates.
- Steam and chlorine accelerate sintering. Additionally, moist atmospheres, overheating, and surface area losses accelerate structural changes in oxide supports.
--Coking
Coking accounts for about 20% of catalyst deactivation, and it is usually related to plugging. Namely, the carbonaceous and other materials in the catalyst pores deposit, decreasing the pore size and preventing the reactant molecules from diffusing into the pore.
Usually, these carbonaceous deposits can be removed by gasification with water vapor or hydrogen, and we acquire CH4, CO, and COx, respectively. So, coking deactivation is a reversible process. Figure 2. is a schematic illustration of coke deposition on unmodified and metal-modified HZSM-5 catalysts.
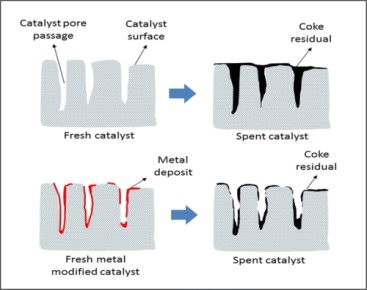
[2]
Figure 2. Coke Deposition
--Others
There are many other useful approaches to prevent catalyst deactivation.
- Choose the Right Catalyst
Choosing the right catalyst for the specific application is critical in preventing deactivation. Different catalysts have varying degrees of stability and resistance to deactivation. Therefore, it is important to select a catalyst that is suitable for the specific process conditions. The catalyst design also matters. You can change the surface area, pore size, and pellet size to prevent catalyst poisoning.
- Keep the Catalyst Clean
One of the main reasons for catalyst deactivation is the accumulation of contaminants on its surface. These impurities can come from the feedstock or from the surrounding environment. To prevent this from happening, it is essential to periodically purge the system or filter the feedstock.
- Avoid High Temperatures
Catalysts can be sensitive to high temperatures, which can lead to their deactivation. It is crucial to avoid exposing the catalyst to temperatures beyond its safe operating range. You’d better monitor the temperature of the system and adjust the process accordingly.
- Monitor Catalyst Activity
Monitoring the activity of the catalyst can help to detect any changes in its performance. This can be achieved by regularly measuring the reaction rate or by conducting periodic catalyst testing. By monitoring the activity of the catalyst, any issues can be identified early, and corrective action can be taken to prevent deactivation.
Conclusion
In a word, please follow the above steps to fight against poisoning, sintering, and coking, which are the main causes of catalyst deactivation. Also, you’d better pay attention to the operating conditions and the proper selection, use, and maintenance of the catalyst. Thus, the life of catalysts can be prolonged, resulting in improved efficiency and reduced costs in industrial processes.
Stanford Advanced Materials (SAM) provides all kinds of precious metal catalysts at affordable prices. Other precious metal products including precious metal crucibles and precious metal wires are also available. Please check our site for more information.
Reference:
[1] Philipp Wachter, Christian Gaber, Juraj Raic, Martin Demuth, Christoph Hochenauer, (2021). Experimental investigation on H2S and SO2 sulfur poisoning and regeneration of a commercially available Ni-catalyst during methane tri-reforming [Photograph]. https://www.sciencedirect.com/science/article/abs/pii/S0360319920340921
[2] Balasundram, Vekes & Ibrahim, Norazana & Kasmani, Rafiziana & Isha, Ruzinah & Abd Hamid, Mohd Kamaruddin & Hasbullah, Hasrinah. (2022). Catalytic upgrading of biomass-derived pyrolysis vapour over metal-modified HZSM-5 into BTX: A comprehensive review [Photograph]. https://www.researchgate.net/publication/343461067_Catalytic_upgrading_of_biomass-derived_pyrolysis_vapour_over_metal-modified_HZSM-5_into_BTX_a_comprehensive_review