Everything You Need to Know About Titanium Oxides
Introduction
Titanium oxides are compounds consisting of titanium and oxygen, known for their remarkable properties and wide-ranging applications. These oxides are primarily represented by two forms: titanium dioxide (TiO₂) and titanium monoxide (TiO). This article delves into the characteristics, synthesis methods, applications, and environmental impact of titanium oxides.
Types of Titanium Oxides
1. Titanium Dioxide (TiO₂)
TiO₂ is a white, odorless powder with a high refractive index and strong UV light absorption. It exhibits photocatalytic activity and chemical stability, and is non-toxic.
TiO₂ exists in three main polymorphs—anatase, rutile, and brookite. Anatase and rutile are the most common, with rutile being thermodynamically stable and anatase transforming to rutile upon heating.

2. Titanium Monoxide (TiO)
TiO is less common, with a metallic luster and electrical conductivity. It has a rock salt structure and is often used in specialized applications such as thin films and coatings.
Synthesis of Titanium Oxides
1. Titanium Dioxide (TiO₂)
- The sulfate process for producing TiO₂ involves reacting ilmenite (FeTiO₃) with sulfuric acid, which produces titanyl sulfate. This compound is then hydrolyzed and calcined to yield titanium dioxide.
- Another method, known as the chloride process, involves the chlorination of ilmenite or rutile at high temperatures to form titanium tetrachloride (TiCl₄), which is subsequently oxidized to produce TiO₂.
- A more modern approach is the sol-gel method, where titanium alkoxides undergo hydrolysis and polymerization, followed by drying and calcination. This process results in TiO₂ nanoparticles with controlled size and morphology.
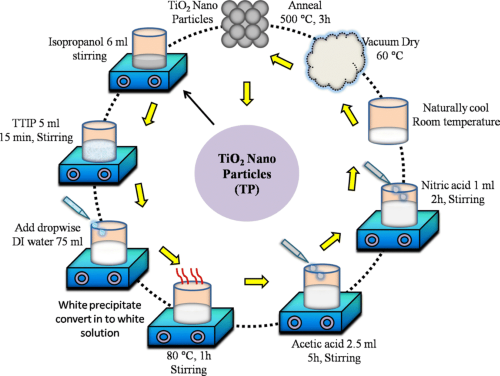 [1]
[1]
2. Titanium Monoxide (TiO)
The synthesis of titanium monoxide (TiO) typically employs reduction methods. TiO is commonly produced by reducing TiO₂ with hydrogen or through the direct combination of titanium and oxygen under carefully controlled conditions.
Applications of Titanium Oxides
1. Titanium Dioxide (TiO₂)
- Pigments: TiO₂ is the most widely used white pigment due to its brightness and opacity. It is used in paints, coatings, plastics, paper, and inks.
- Sunscreens and Cosmetics: Due to its strong UV absorption, TiO₂ is a key ingredient in sunscreens and other cosmetic products, providing protection against harmful UV radiation.
- Photocatalysis: TiO₂'s photocatalytic properties make it useful in environmental applications such as air and water purification, self-cleaning surfaces, and antibacterial coatings.
- Electronics: TiO₂ is used in the production of electronic components, such as varistors and capacitors, due to its dielectric properties.
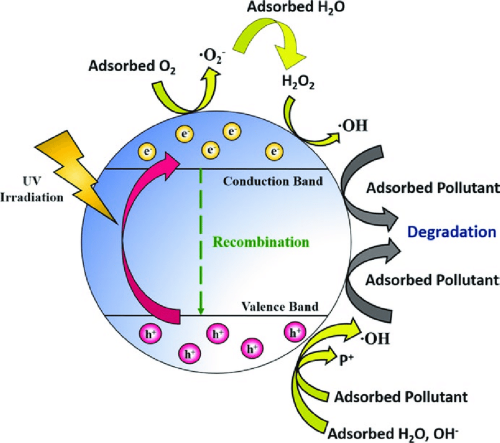 [2]
[2]
2. Titanium Monoxide (TiO)
Thin Films and Coatings: TiO is used in the production of thin films for applications in optical coatings, semiconductors, and sensors. Its electrical conductivity and thermal stability make it suitable for these purposes.
Environmental Impact and Safety
Environmental Impact: While TiO₂ is generally considered safe for human health and the environment, its widespread use raises concerns about nanoparticle pollution. TiO₂ nanoparticles can enter water bodies, potentially impacting aquatic life. Therefore, regulations and guidelines are in place to manage its production and disposal.
Human Health: TiO₂ is considered non-toxic, and its use in food, cosmetics, and pharmaceuticals is regulated to ensure safety. However, inhalation of TiO₂ dust may pose respiratory risks, highlighting the importance of proper handling and protective measures in industrial settings.
Photocatalytic Activity: The photocatalytic properties of TiO₂ can lead to the generation of reactive oxygen species (ROS), which may have both beneficial and detrimental effects. In environmental applications, ROS can degrade pollutants, but excessive exposure to ROS can cause oxidative stress in living organisms.
Future Prospects and Research
Research is ongoing to develop advanced TiO₂-based materials with enhanced properties for applications in energy storage, photovoltaics, and photocatalysis. Innovations include doping TiO₂ with other elements to improve its efficiency and exploring new synthesis methods for better control over particle size and morphology.
Efforts are being made to develop more sustainable and environmentally friendly methods for producing and utilizing titanium oxides. This includes the use of green chemistry principles, recycling of TiO₂ waste, and improving the efficiency of photocatalytic processes.
Conclusion
Titanium oxides, particularly TiO and TiO₂, play a crucial role in various industries. TiO, with its metallic luster and electrical conductivity, is primarily used in specialized applications such as thin films and coatings. While, TiO₂, known for its high refractive index, strong UV light absorption, photocatalytic activity, and chemical stability, finds extensive use in pigments, sunscreens, cosmetics, photocatalysis, and electronics.
As technology advances, titanium oxides are poised to remain at the forefront of material science and industrial applications. Stanford Advanced Materials (SAM) provides high-quality Titanium products at competitive prices. We offer Photocatalytic Nano Titanium Dioxide Powder, Nano Titanium Dioxide Powder for Lithium Batteries, Nano Titanium Dioxide Powder for Ceramics, as well as Anatase and Rutile forms of Titanium Dioxide. For more information, please check our homepage.
Aspect | Titanium Monoxide (TiO) | |
Properties | White, odorless powder, high refractive index, strong UV light absorption, photocatalytic activity, chemical stability, non-toxic. | Metallic luster, electrical conductivity, rock salt structure. |
Synthesis Methods | Sulfate Process: Reacting ilmenite (FeTiO₃) with sulfuric acid, hydrolyzing and calcining titanyl sulfate to yield TiO₂. Chloride Process: Chlorination of ilmenite or rutile to form TiCl₄, then oxidized to produce TiO₂. Sol-Gel Method: Hydrolysis and polymerization of titanium alkoxides, followed by drying and calcination to obtain TiO₂ nanoparticles. | Reduction Methods: Reducing TiO₂ with hydrogen or direct combination of titanium and oxygen under controlled conditions. |
Applications | Pigments: Paints, coatings, plastics, paper, and inks. Sunscreens and Cosmetics: UV protection. Photocatalysis: Air and water purification, self-cleaning surfaces, antibacterial coatings. Electronics: Varistors, capacitors. | Thin Films and Coatings: Optical coatings, semiconductors, sensors. |
Reference:
[1] Pawar, Vani & Kumar, Manish & Dubey, Pawan & Singh, Manish Kumar & Sinha, Ask & Singh, Prabhakar. (2019). Influence of synthesis route on structural, optical, and electrical properties of TiO2. Applied Physics A. 125. 10.1007/s00339-019-2948-3.
[2] Leong, Kah & Ching, Sim & Pichiah, Saravanan & Ibrahim, S. (2016). Light Driven Nanomaterials for Removal of Agricultural Toxins.