What is Cobalt Used in Everyday Life
Introduction
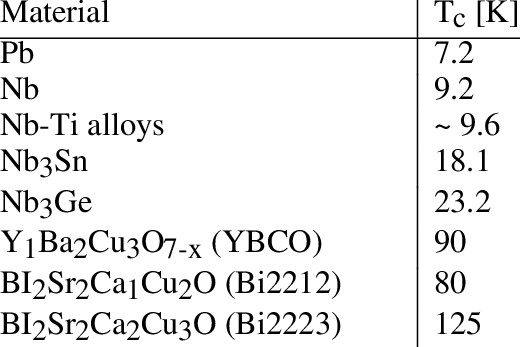
Cobalt, denoted by the chemical symbol Co with an atomic number of 27 and a melting point of 1495℃, stands out as a high-melting-point metal boasting ductility, ferromagnetism, and a silver-gray metallic luster. Despite its scarcity, cobalt plays a pivotal role in various industrial applications, earning it the monikers "industrial monosodium glutamate" and "industrial tooth." This article explores the multifaceted uses of cobalt in everyday life, emphasizing its significance as a strategic resource.
Cobalt Resources and Reserves
Cobalt resources are predominantly associated with copper-cobalt ore, nickel-cobalt ore, arsenic-cobalt ore, and pyrite deposits. While independent cobalt minerals are rare, seabed manganese nodules emerge as a promising long-term resource for cobalt. Recognized as an important strategic resource, cobalt scarcity underscores its value in diverse industries.

Applications of Cobalt in Various Forms
Traditional Uses:
- Battery Materials: Cobalt has a significant presence in battery materials, contributing to the efficiency and performance of various batteries.
- Super Heat-Resistant Alloys: Its high-temperature resistance makes cobalt a crucial component in super heat-resistant alloys.
- Tool Steels and Hard Alloys: Cobalt enhances the hardness and durability of tool steels and hard alloys.
- Magnetic Materials: The ferromagnetic properties of cobalt make it indispensable in the production of magnetic materials.
Compound Forms:
- Catalysts and Desiccants: Cobalt, in compound forms, serves as catalysts and desiccants in chemical processes.
- Reagents: It functions as a reagent, contributing to various chemical reactions.
- Pigments and Dyes: Cobalt compounds find application in the production of pigments and dyes, adding color to various products.
Radioactive Application:
- Cobalt-60: This radioactive isotope is extensively used in biochemistry for activation analysis, electroplating, corrosion, catalysis for tracer research, and medical treatments such as radiological examinations and therapies.
Industrial Applications
Owing to its remarkable properties—high-temperature resistance, corrosion resistance, and magnetic performance—cobalt finds widespread use in diverse industries. Aerospace, machinery manufacturing, electrical and electronic sectors, chemical, and ceramic industries all rely on cobalt as a fundamental raw material. It contributes significantly to the production of high-temperature alloys, hard alloys, ceramic pigments, catalysts, and batteries.
Magnetic Superiority of Cobalt
Cobalt stands out among metals due to its exceptional magnetic properties. Maintaining magnetism with a single magnetization sets cobalt apart. With a Curie point—a temperature of magnetic loss—reaching 1150℃, cobalt surpasses iron and nickel in this regard. Cobalt's application in high-performance magnetic materials underscores its magnetically superior characteristics. In comparison to conventional magnetic steel, cobalt steel exhibits significantly lower magnetism loss during vibration, making it an ideal choice for various applications.

Conclusion
In conclusion, cobalt's versatility and unique properties position it as an indispensable element in everyday life and various industries. From enhancing the performance of batteries to contributing to the production of high-performance magnetic materials, cobalt's impact is far-reaching. As industries evolve, the strategic significance of cobalt in materials science, technology, and manufacturing remains paramount.
Stanford Advanced Materials (SAM), with over 20 years of experience, stands as a reliable supplier offering a spectrum of rare earth metals and their compounds. For inquiries and orders related to cobalt or other materials, feel free to contact SAM, which provides excellent quality and competitive prices.