Inversion Temperature Explained
Introduction
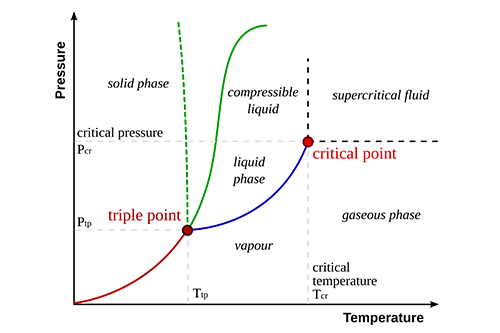
The concept of inversion temperature is crucial in the fields of thermodynamics, chemistry, and physics, particularly in understanding the behavior of gases and vapors. It refers to the temperature at which the specific volume of a gas becomes equal to its specific volume when it is in its liquid phase. In simpler terms, it is the temperature at which a gas can be liquefied by increasing pressure, without the need for a reduction in temperature.
Definition of Inversion Temperature
In the context of gases, the inversion temperature is defined as the temperature above which a gas cannot be liquefied by pressure alone. Below this temperature, increasing the pressure on the gas can cause it to condense into a liquid.
The term is often associated with real gases (gases that exhibit non-ideal behavior) as opposed to ideal gases. Real gases deviate from the ideal gas law, especially at high pressures and low temperatures. The inversion temperature is a characteristic property of each gas and is a key factor in determining whether a gas can be liquefied through compression.
Mathematical and Thermodynamic Explanation
The concept of inversion temperature can be understood in terms of the van der Waals equation, which is an equation of state for real gases. This equation accounts for the non-ideal behavior of gases by incorporating the attractive forces between molecules and the finite volume of the gas molecules.
For a real gas, the van der Waals equation is expressed as:
(P+a /V^2)*(V−b)=RT
Where:
- P = pressure
- V = volume
- a = van der Waals constant for attraction between molecules
- b = van der Waals constant for the volume occupied by molecules
- R = gas constant
- T = temperature
At temperatures above the inversion temperature, the gas molecules have enough kinetic energy to overcome intermolecular attractive forces, preventing liquefaction even when pressure is applied. However, below this temperature, the attractive forces are strong enough that the gas can be liquefied upon compression.
The inversion temperature is a critical point where the nature of the gas changes from being easily compressible into a liquid to a state where no compression alone can cause condensation.
Factors Affecting Inversion Temperature
Several factors influence the inversion temperature of a gas:
Molecular Structure:
The molecular composition and structure of a gas play a crucial role in determining its inversion temperature. Gases with larger, more complex molecules (such as carbon dioxide) tend to have lower inversion temperatures.
Intermolecular Forces:
Gases with stronger intermolecular forces (such as hydrogen bonding or van der Waals forces) typically have lower inversion temperatures, as the molecules are more likely to condense into a liquid phase at lower temperatures.
Atomic Size:
Larger atoms or molecules with more electrons can experience stronger London dispersion forces, which increase the likelihood of liquefaction at lower temperatures, thus lowering the inversion temperature.
Inversion Temperature and Liquefaction of Gases
The inversion temperature is of particular importance in the study of the liquefaction of gases. For instance, carbon dioxide (CO₂) has an inversion temperature of around 31°C. This means that at temperatures above 31°C, CO₂ cannot be liquefied by increasing pressure alone, regardless of how much pressure is applied. However, at temperatures below 31°C, CO₂ can be compressed into a liquid.
This principle is critical in various industrial processes, such as:
· Refrigeration: In refrigeration cycles, gases like ammonia and Freon are used. Understanding the inversion temperature helps design systems that maintain the gas in the correct state (liquid or gas) at the desired pressures and temperatures.
· Gas Liquefaction: The liquefaction of natural gases (such as methane) is a process where gases are cooled and compressed. The inversion temperature helps engineers determine the operating conditions for liquefaction.
· For more information, please check Stanford Advanced Materials (SAM).
Inversion Temperature in Practical Applications
Cryogenics:
Cryogenics is the science of producing and studying very low temperatures. The inversion temperature is vital for understanding and achieving the liquefaction of gases in cryogenic processes. For example, liquefied oxygen and nitrogen are produced at extremely low temperatures, well below their inversion temperatures.
Natural Gas Processing:
In the natural gas industry, gases like methane are cooled and compressed for transportation. The inversion temperature informs engineers about how to manipulate pressure and temperature to efficiently liquefy or transport these gases.
The Joule-Thomson Effect:
The inversion temperature is connected to the Joule-Thomson effect, which describes how a gas expands or contracts when allowed to expand through a valve or porous plug. For most gases, at temperatures above the inversion temperature, expansion leads to heating, while at temperatures below it, expansion causes cooling. This principle is used in refrigeration and gas expansion technologies.
Air Conditioning:
Understanding the inversion temperature of refrigerants used in air conditioners allows engineers to design systems that efficiently cool and compress the refrigerant for heat exchange.
Frequently Asked Questions
What is the inversion temperature?
The inversion temperature is the temperature above which a gas cannot be liquefied by pressure alone. Below this temperature, increasing the pressure can cause a gas to condense into a liquid. It is a characteristic property of each gas and depends on its molecular structure and intermolecular forces.
Why is the inversion temperature important in gas liquefaction?
The inversion temperature determines whether a gas can be liquefied by increasing pressure. For gases with a temperature above their inversion temperature, no amount of pressure will cause condensation. Understanding this temperature helps in the design of industrial processes like refrigeration, natural gas liquefaction, and cryogenics.
How does the inversion temperature affect the Joule-Thomson effect?
The inversion temperature is linked to the Joule-Thomson effect, which describes how a gas changes temperature when it expands or contracts. If the gas is above its inversion temperature, expansion causes heating; if below, expansion causes cooling. This principle is crucial in applications such as refrigeration and air conditioning.
What factors influence the inversion temperature of a gas?
Several factors affect the inversion temperature, including the molecular structure of the gas, the strength of intermolecular forces (such as hydrogen bonding or van der Waals forces), and the atomic size. Larger, more complex molecules or gases with stronger intermolecular forces tend to have lower inversion temperatures.
Can the inversion temperature be used to select gases for industrial applications?
Yes, the inversion temperature plays a key role in selecting gases for industrial applications. For example, in refrigeration and cryogenics, choosing a gas with a suitable inversion temperature ensures efficient operation under the desired pressure and temperature conditions. It also helps in optimizing processes like gas liquefaction and transportation.