What Are Biomedical Metals?
Introduction
Biomedical metals are essential in modern medicine. They help diagnose, treat, repair, or replace damaged tissues in the human body. These metals are used to repair and replace hard tissues like bones and teeth. They also treat cardiovascular and soft tissues and are involved in manufacturing artificial organs.
The global pharmaceutical market is expanding, leading to a wider use of biomedical metals. These materials include various metals and alloys. They are known for their high mechanical strength and durability, which makes them ideal for load-bearing implants. To function effectively over long periods, biomedical metals must possess excellent mechanical and physical properties. They also need superior corrosion resistance, biocompatibility, and ease of processing.
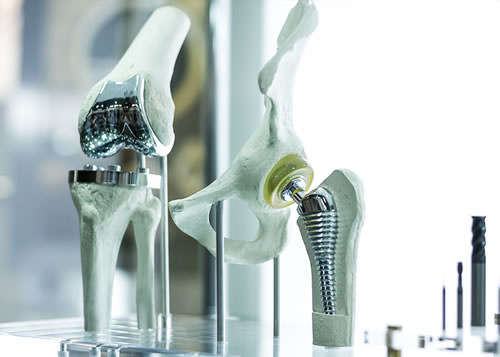
Types of Biomedical Metals
Tantalum
Tantalum is a refractory metal with a solid-core cubic structure. It has strong chemical stability and resistance to corrosion. In the physiological environment, tantalum forms a stable passivated film on its surface. This film protects it from corrosion and ensures biocompatibility.
Tantalum is valuable in orthopedics and dentistry. It integrates well with bone tissue, promoting new bone formation around it. Tantalum is used to make bone plates, screws, and implant roots. Its unique properties also make it suitable for surgical sutures and muscle defect repairs. Its high electronegativity provides excellent antithrombotic properties, beneficial for endovascular stents and artificial hearts. Additionally, tantalum’s isotopes are used in radiation therapy due to their effective radiographic properties.
Niobium
Niobium is another refractory metal with a melting point of 2467°C and a body-centered cubic structure. It shares many properties with tantalum, including chemical stability and corrosion resistance. Niobium also has good biocompatibility with human tissues.
Niobium is used to manufacture medical implants like intramedullary nails. However, its use is limited due to economic and sourcing issues. Despite these limitations, niobium remains valuable in specific biomedical applications where its properties are particularly advantageous.
Zirconium
Zirconium has a melting point of 1952°C and features a dense cubic structure at room temperature. Above 862°C, it transitions to a body-centered cubic structure. Zirconium shares similar chemical properties with titanium, including its ability to form a protective passivated film when exposed to oxygen and hydrogen at high temperatures.
Zirconium is highly corrosion-resistant and has excellent processing properties. Medical-grade zirconium is often used with pure titanium in clinical settings. However, its use is somewhat limited due to its high cost compared to other biomedical metals. Zirconium can be processed into plates, belts, and wires for use in implants and other medical devices.
Titanium
Titanium is a lightweight and strong metal with a melting point of 1885°C. It forms a stable oxide layer, which makes it highly resistant to corrosion. Titanium integrates well with bone tissue, showing excellent biocompatibility. This property makes it a popular choice for implants, including dental crowns, bone plates, and joint prostheses. Additionally, its low density and high strength-to-weight ratio make it ideal for orthopedic and dental applications.
Stainless Steel
Stainless steel, especially the 316L alloy, is widely used in biomedical applications. It offers excellent strength, corrosion resistance, and affordability. This metal is used in a variety of medical devices such as surgical instruments, stents, and orthopedic implants. Although its biocompatibility is lower compared to titanium or tantalum, its cost-effectiveness and mechanical properties make it a common choice.
Cobalt-Chromium Alloys
Cobalt-chromium alloys, such as Co-Cr-Mo, are known for their high strength, wear resistance, and corrosion resistance. These alloys are commonly used in orthopedic implants, dental prosthetics, and cardiac devices. They are suitable for high-stress applications due to their mechanical properties. However, they are often more rigid than titanium, which can affect their integration with bone.
Magnesium Alloys
Magnesium alloys are becoming promising materials for temporary implants due to their biodegradability. They are used in bone fixation devices that gradually dissolve as the bone heals. Magnesium alloys have good mechanical properties and are lightweight. However, their corrosion rates in the physiological environment need careful management.
Platinum
Platinum is a noble metal known for its excellent biocompatibility and corrosion resistance. It is used in medical devices where biocompatibility is critical, such as certain types of electrodes and implants. Although platinum is less common than titanium or stainless steel due to its high cost, its unique properties make it valuable for specific applications.
Conclusion
Biomedical metals are crucial in modern medical devices and implants because of their unique properties, such as strength, biocompatibility, and corrosion resistance. Each metal offers specific advantages for different medical applications. For instance, titanium integrates well with bone, while tantalum has antithrombotic qualities. Companies like Stanford Advanced Materials (SAM) offer high-quality biomedical metals and alloys. Their expertise and products support advancements in medical technology and contribute to improved patient outcomes.